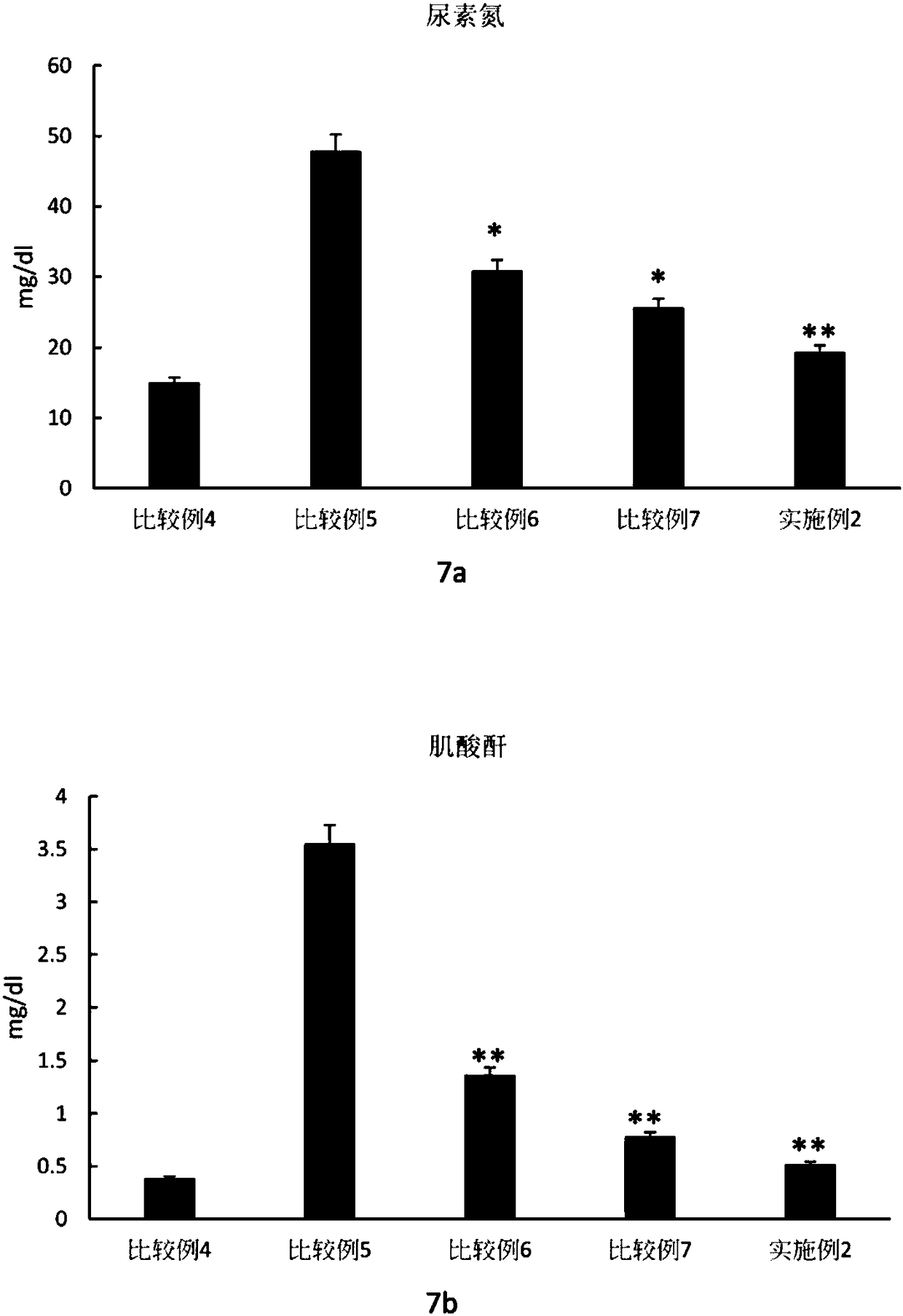
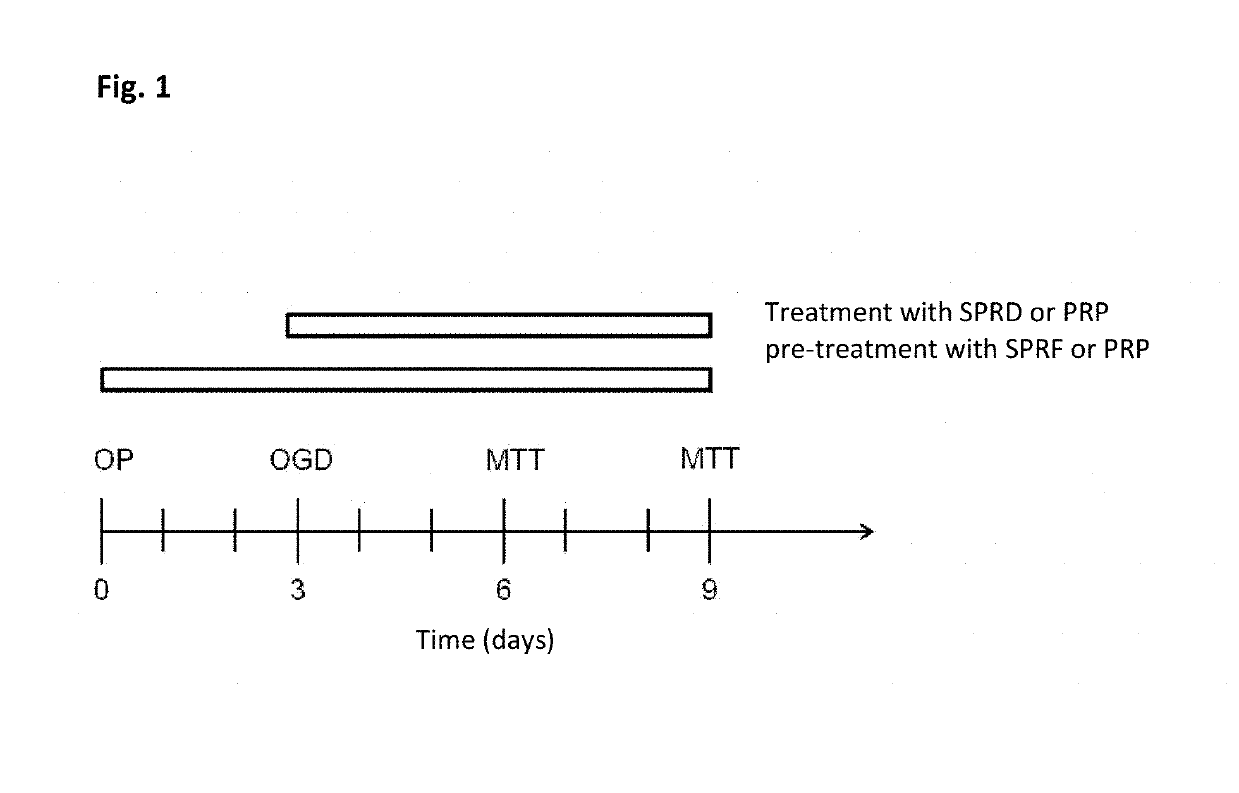
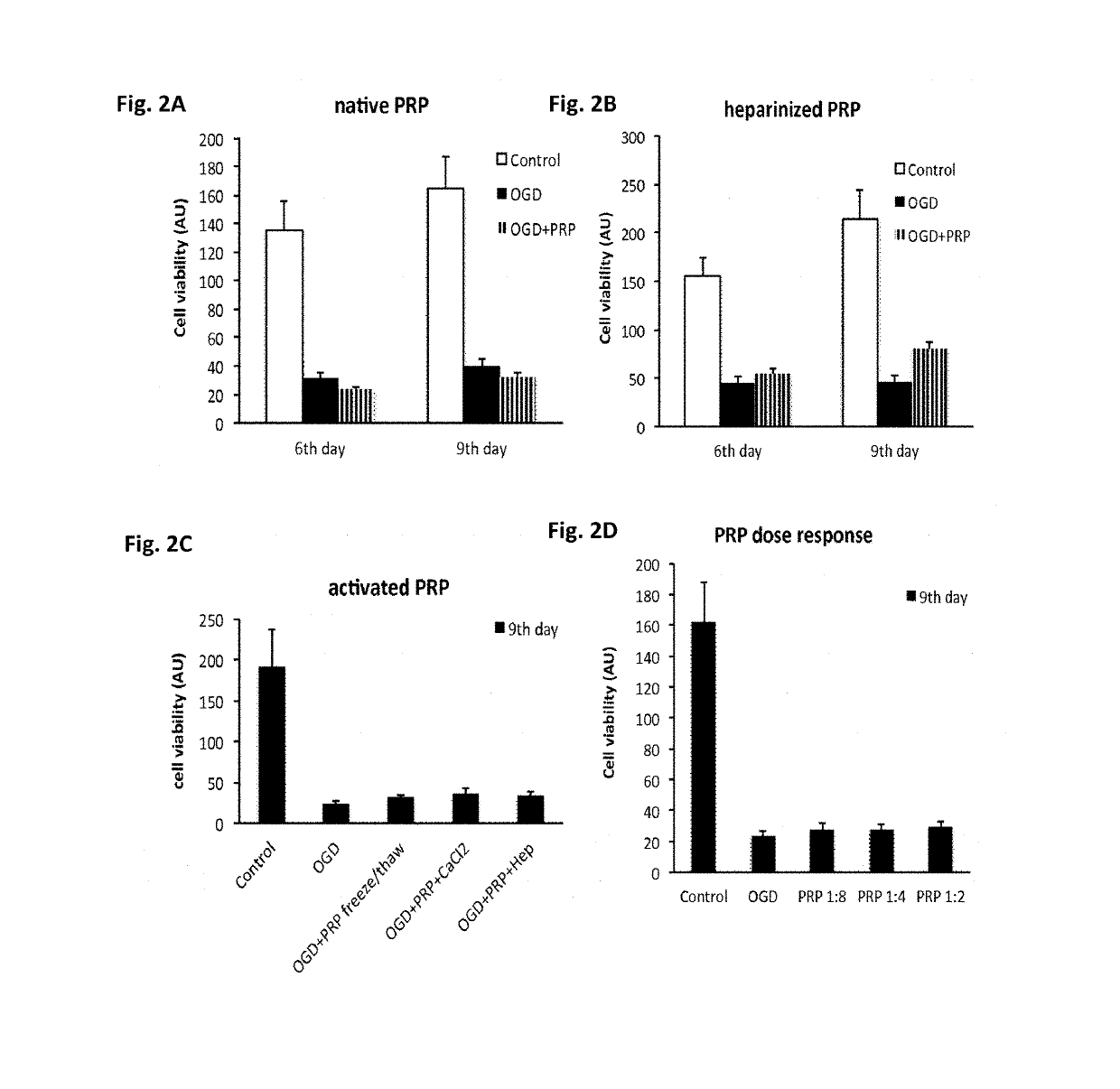
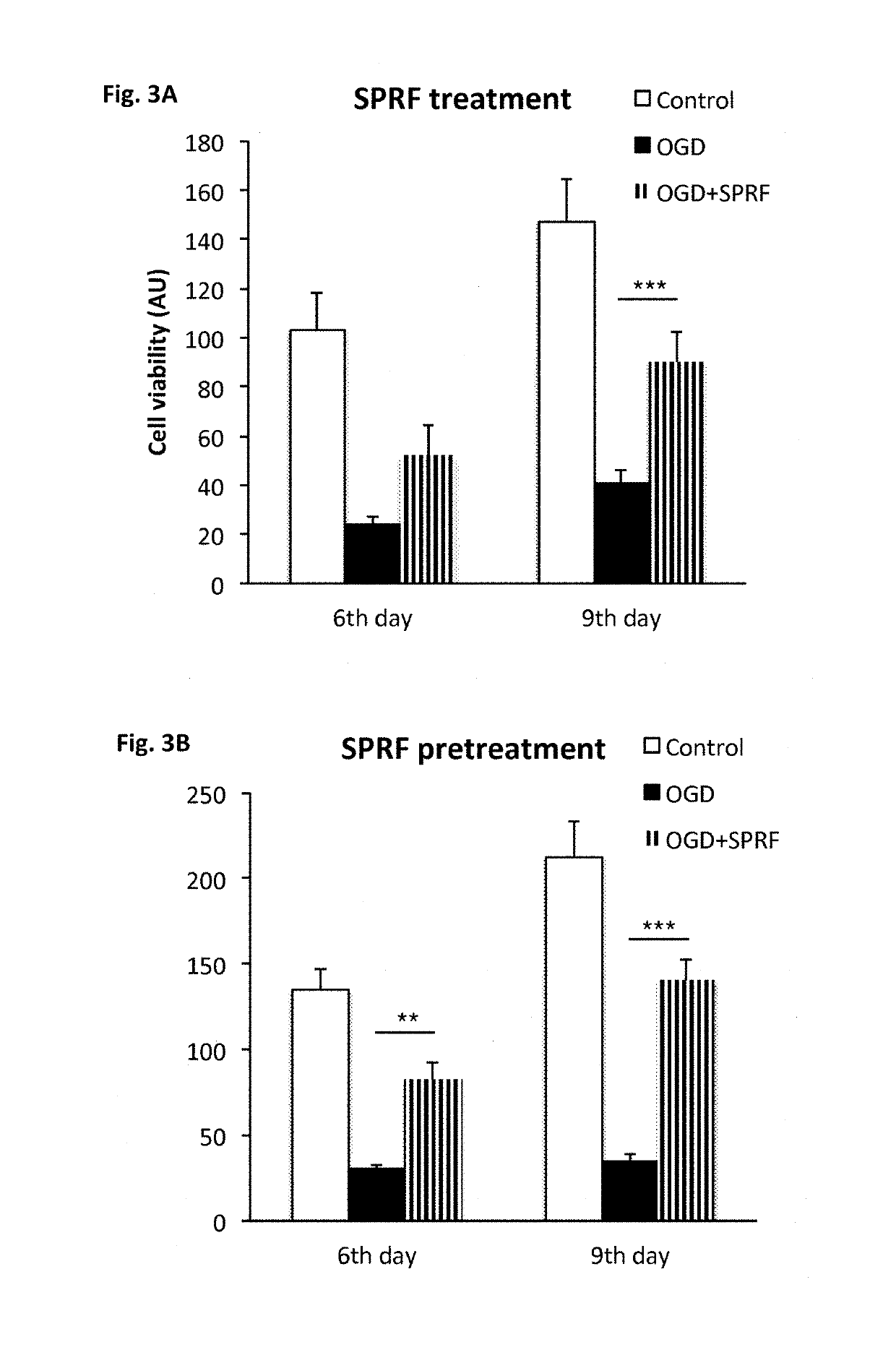
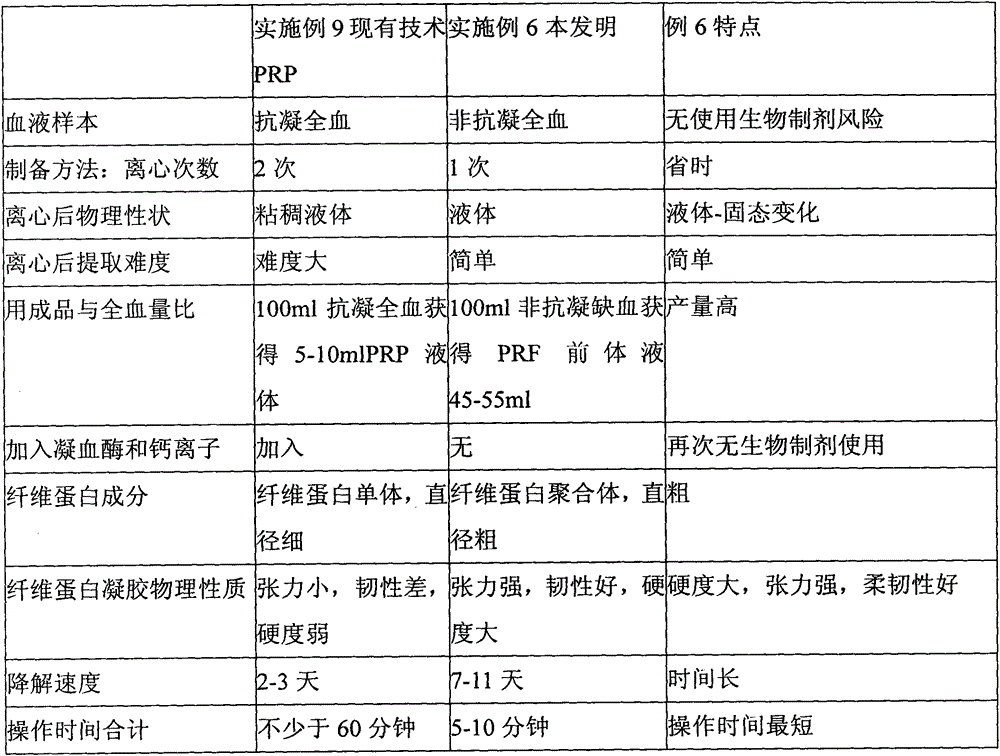
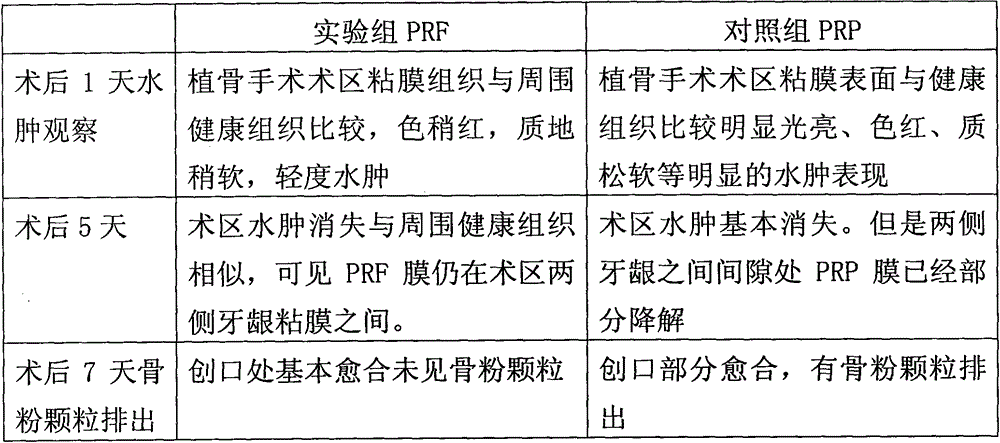
Patents
Literature
34 results about "Platelet-rich fibrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
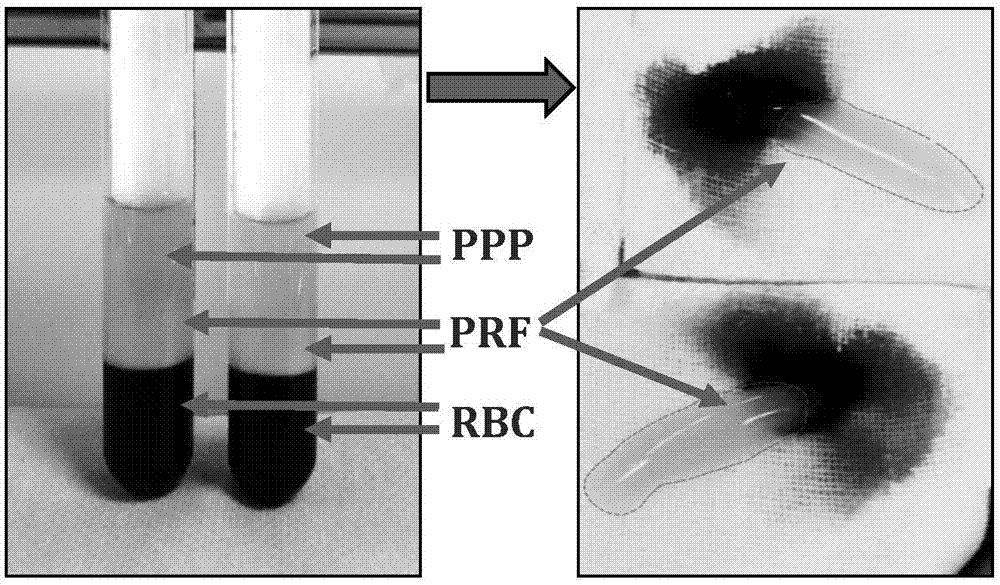
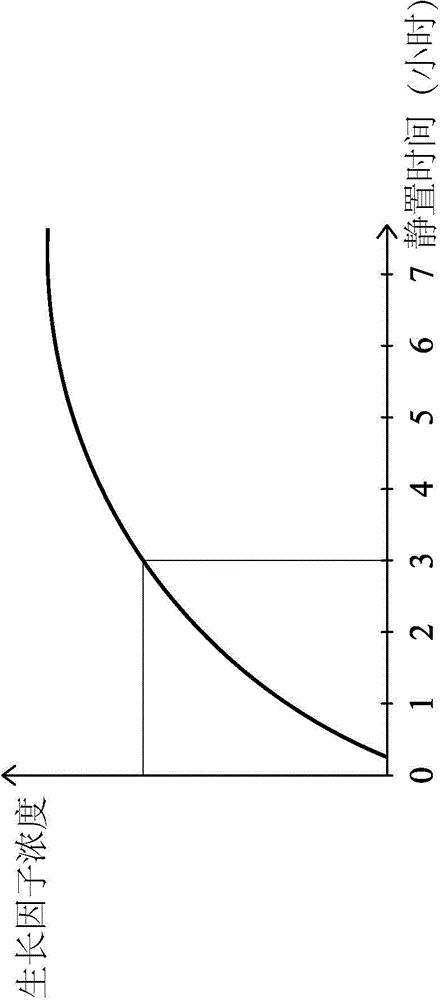
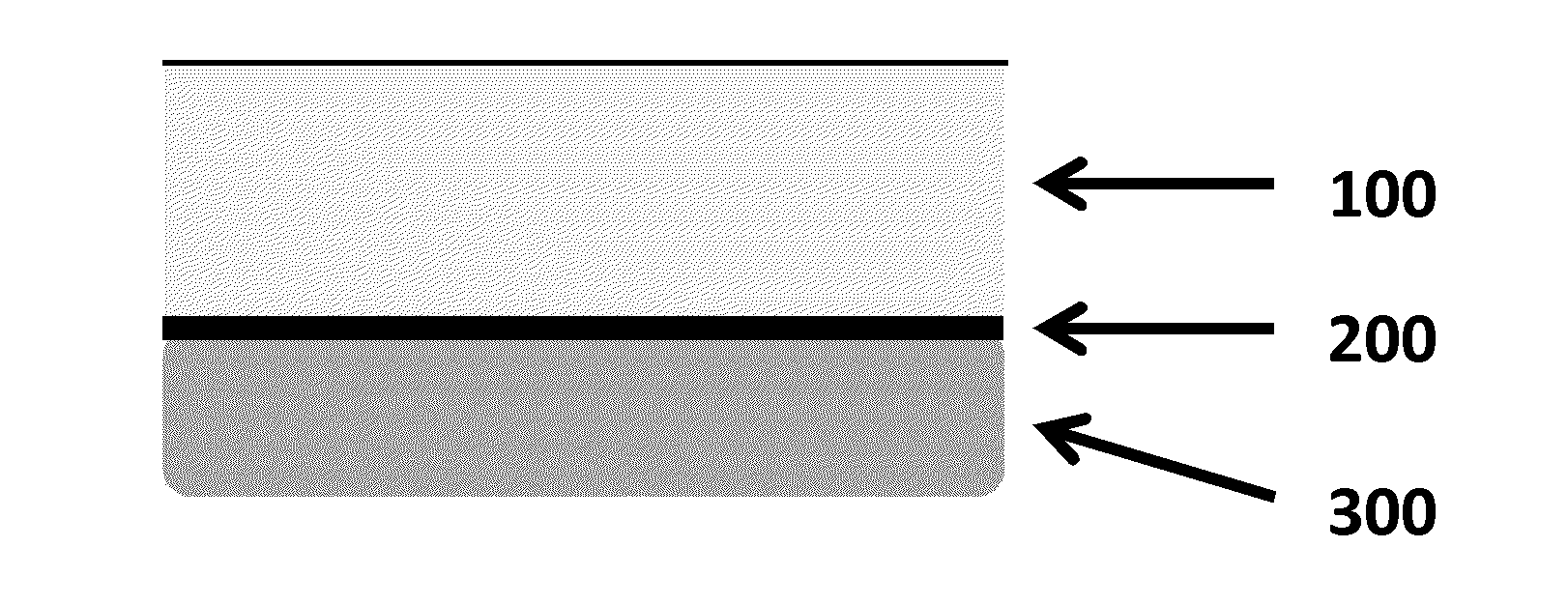
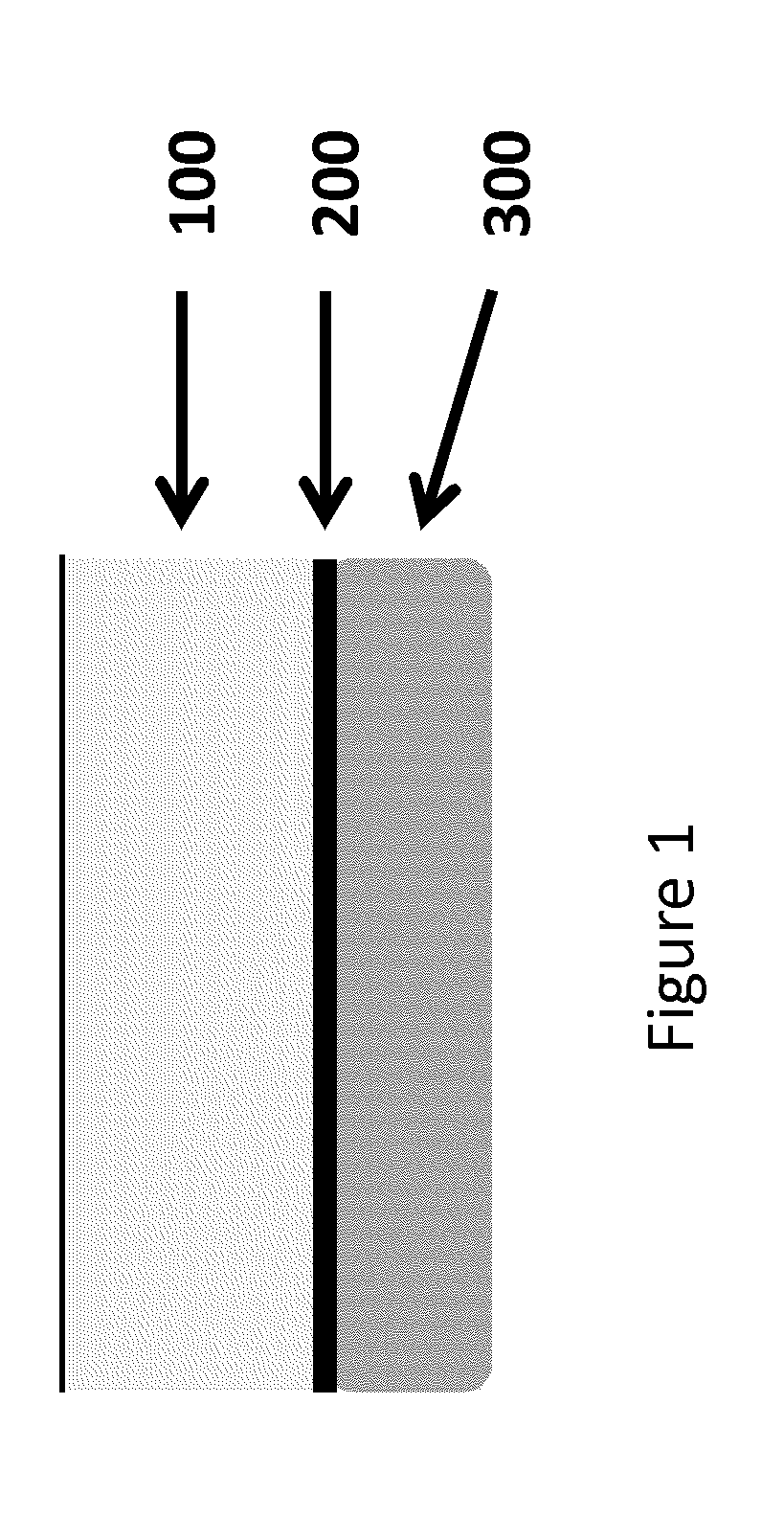
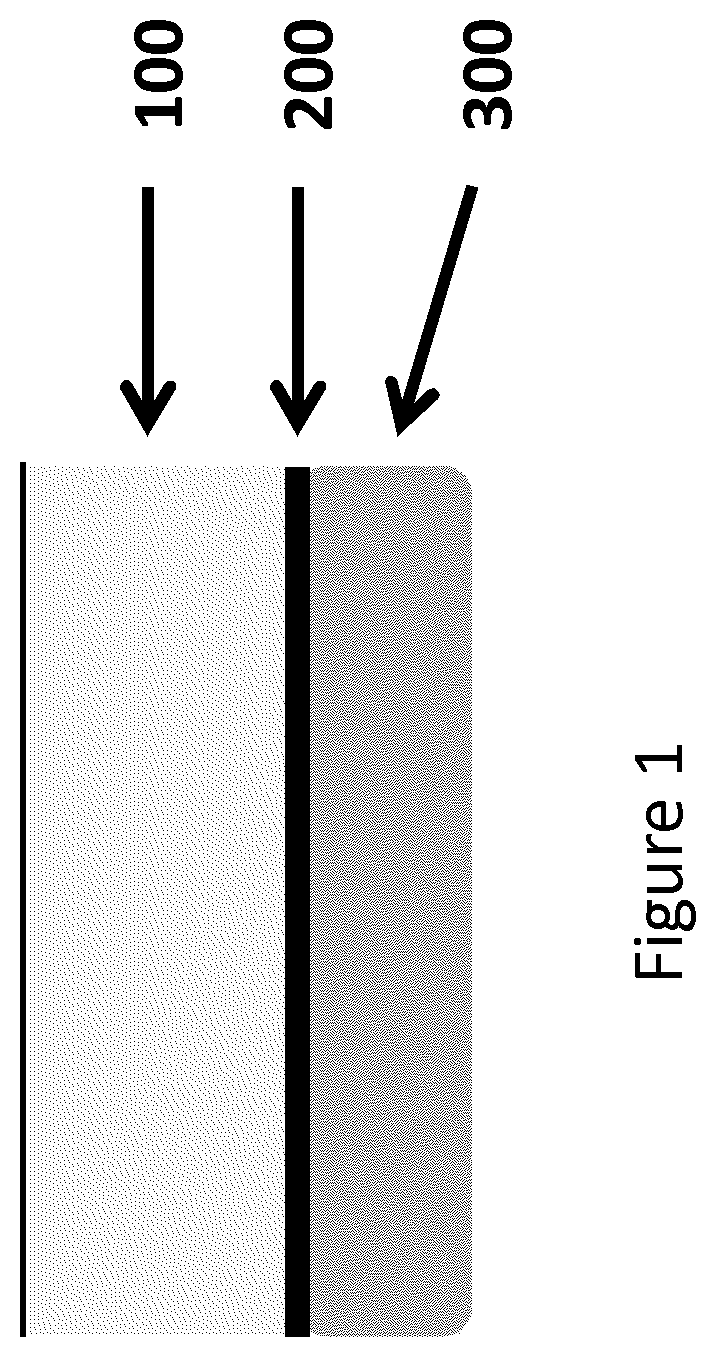
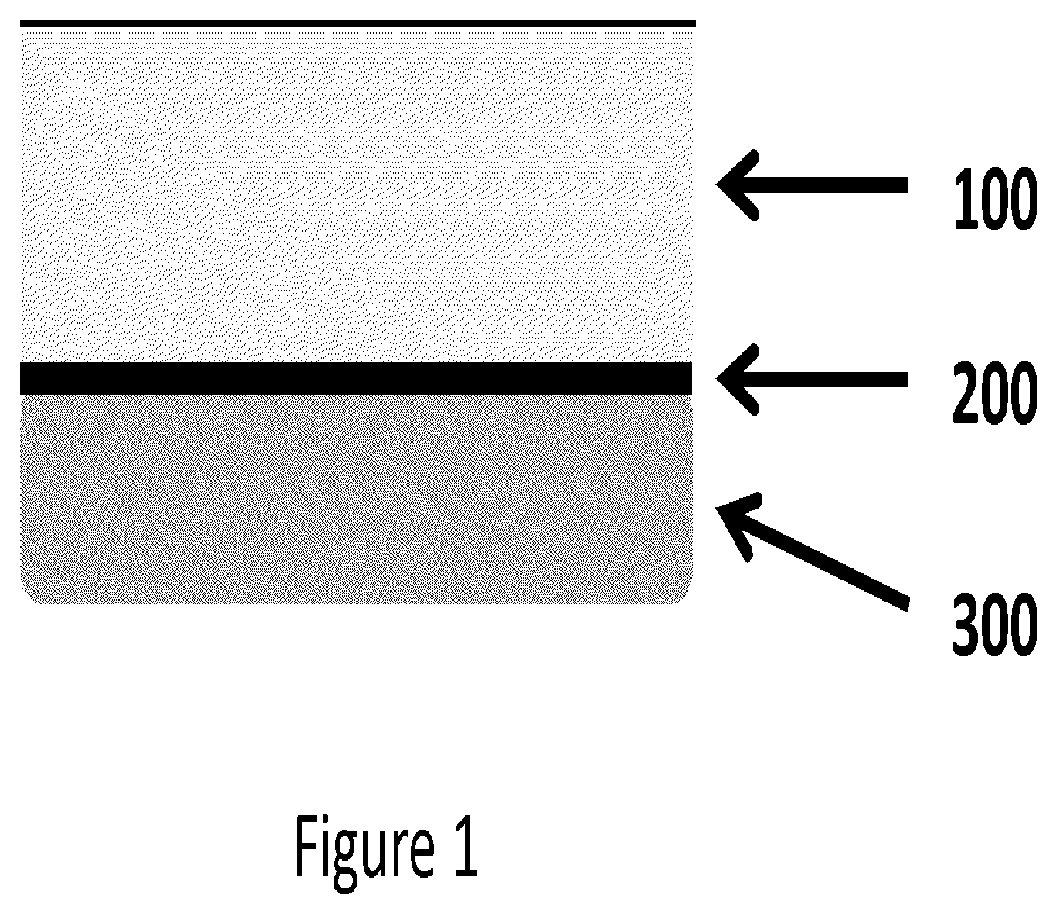
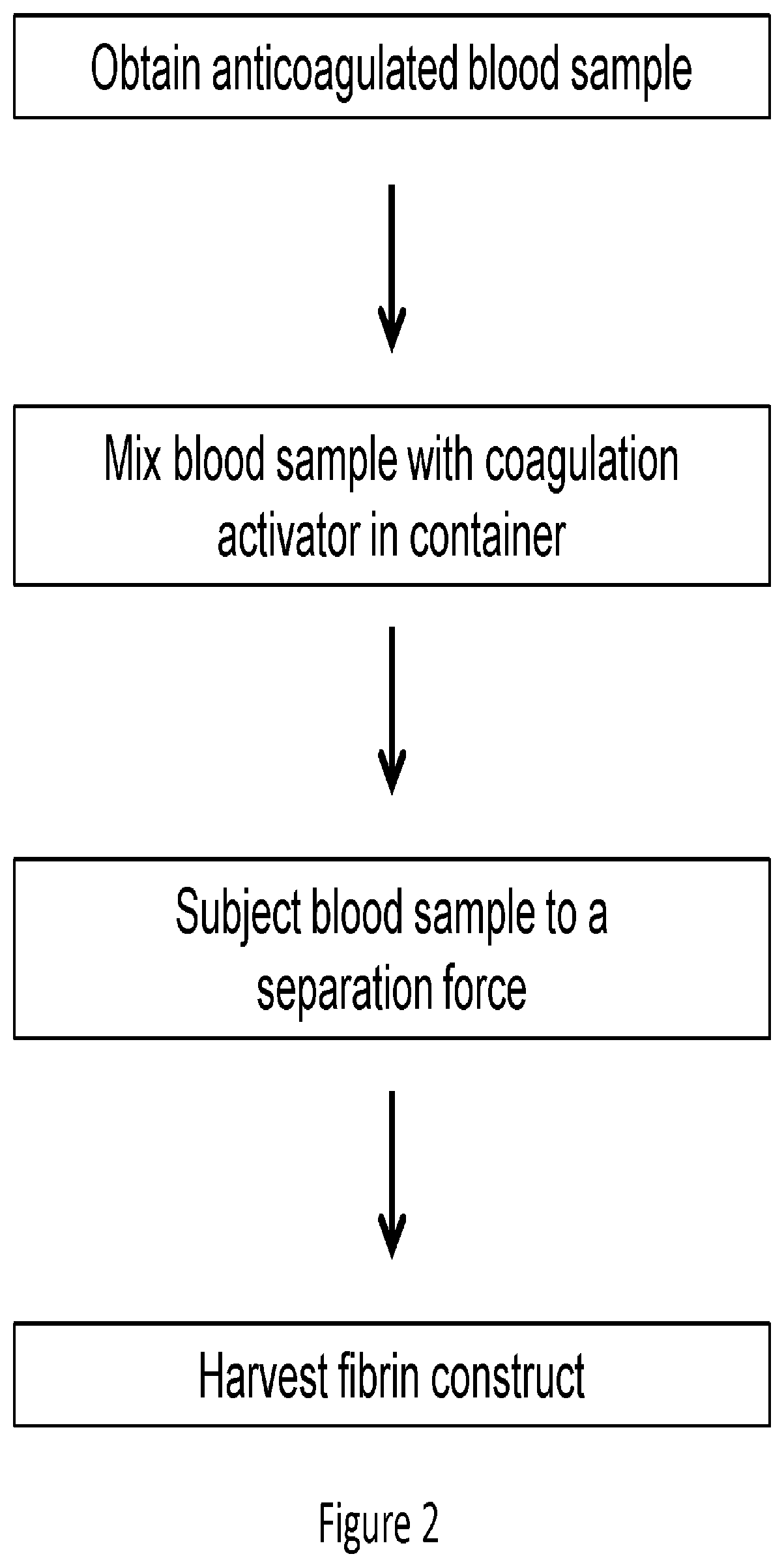
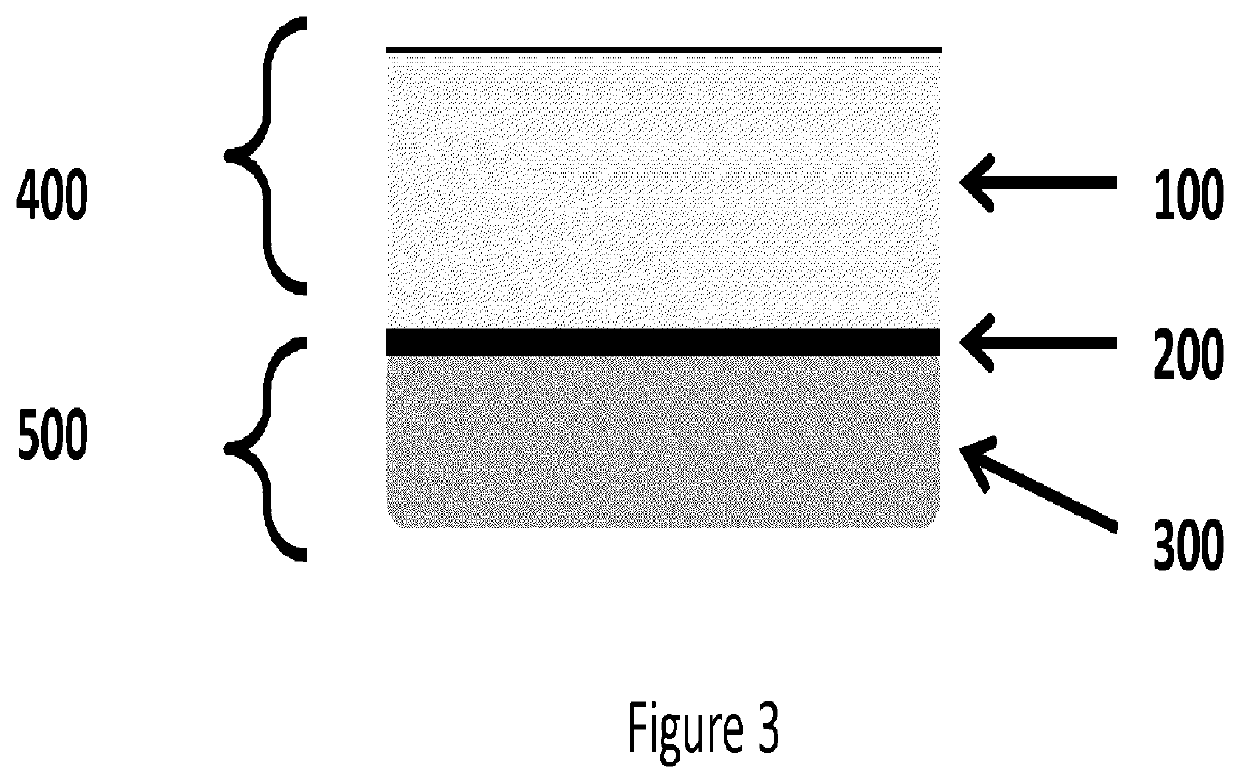
Platelet-rich fibrin (PRF) or leucocyte- and platelet-rich fibrin (L-PRF) is a second-generation PRP where autologous platelets and leucocytes are present in a complex fibrin matrix to accelerate the healing of soft and hard tissue and is used as a tissue-engineering scaffold for endodontics. To obtain PRF, required quantity of blood is drawn quickly into test tubes without an anticoagulant and centrifuged immediately. Blood can be centrifuged using a tabletop centrifuge for at least 10 min at 3000 revolution per minute. The resultant product consists of the following three layers; topmost layer consisting of platelet poor plasma, PRF clot in the middle, and red blood cells at the bottom. PRF is available as a fibrin clot. PRF clot can be removed from the test tube using a sterile tweezer-like instrument. After lifting, the RBC layer attached to the PRF clot can be carefully removed using a sterilized scissor. Platelet activation in response to tissue damage occurs during the process of making PRF release several biologically active proteins including; platelet alpha granules, platelet‑derived growth factor (PGDF), transforming growth factors‑β (TGF‑β), vascular endothelial growth factor (VEGF), and epidermal growth factor. Actually the platelets and leukocyte cytokines are important part in role play of this biomaterial, but the fibrin matrix supporting them is very helpful in constituting the determining elements responsible for real therapeutic potential of PRF. Cytokines are immediately used and destroyed in a healing wound. The harmony between cytokines and their supporting fibrin matrix has much more unique importance than any other platelet derivatives.
Preparation method and use of graft material in double membrane structure
The invention belongs to the field of tissue engineering and biomaterials and discloses a preparation method and use of a graft material in a double membrane structure. The graft material comprises stem cell membrane patch segments and platelet-rich fibrin membrane particles, wherein the stem cells are firstly expanded-cultured, and then induction-cultured by using a membrane induction fluid to obtain a stem cell membrane patch, and the stem cell membrane patch is sheared to prepare the cell membrane patch segment; a platelet-rich fibrin gel is extruded, then liquid content of the extruded platelet-rich fibrin gel is removed to obtain the platelet-rich fibrin membrane, and the platelet-rich fibrin membrane is sheared to form particles; and the cell membrane patch segments and the platelet-rich fibrin membrane particles are mixed according to an optimal proportioning relation of in-vitro screening to prepare the needed graft material. The graft material can be widely applied to tissue repair and regeneration of small-area lesion in oral activity and other parts of the body, and the tissue repair effect is improved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Transplanting material of fat granule tissues compounded with SVFs (Stromal Vascular Fractions) and PRFs (Platelet-Rich Fibrins) as well as preparation method and application thereof
The invention belongs to the technical fields of tissue engineering and biological materials, in particular to a transplanting material of fat granule tissues compounded with SVFs (Stromal Vascular Fractions) and PRF (Platelet-Rich Fibrins) as well as a preparation method and application thereof. The transplanting material comprises fat granules, SVFs and PRF membrane granules. The preparation method comprises the following steps of: extracting fat granules from a patient by adopting liposuction, additionally extracting SVFs obtained by fat granule digestion from the same patient and extracting PRF membrane granules obtained by blood centrifuging from the same patient; mixing the SVFs with the PRF membrane granules; incubating at the temperature 37 DEG C for 10 minutes; and mixing the fatgranules, the SVFs and the PRF membrane granules to obtain the transplanting material of fat granule tissues compounded with the SVFs and the PRFs. The material can be applied to soft tissue molding and soft tissue defect repairing; by adopting the material, the absorption of transplanted fat tissues is effectively reduced, and the fat transplanting effect is improved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Serum fraction of platelet-rich fibrin
ActiveUS20140227241A1AvoidingEasy to solveBiocideInanimate material medical ingredientsSerum igeAnticoagulant
The invention provides for a method of preparing an isolated serum fraction of platelet rich fibrin (PRF), comprising the steps ofa. providing platelet rich plasma (PRP) without the addition of an anticoagulant;b. clotting the PRP to obtain a coagel of PRF; andc. separating the coagel to isolate the serum fraction which comprises an activated platelet releasate;and further provides for the isolated serum fraction obtained by such method, and its medical use.
Owner:LACERTA TECH
Amplification culture method for cloning adipose mesenchymal stem cells
InactiveCN106978396AStrong clonogenicityPromotes damage repairSkeletal/connective tissue cellsCell culture active agentsMesenchymal stem cellBrown adipose tissue
The invention discloses an amplification culture method for cloning adipose mesenchymal stem cells. The amplification culture method includes four steps of (1), extracting platelet-rich fibrin glue; (2), preparing special complete culture media; (3), carrying out isolated culture and subculture on primary adipose mesenchymal stem cells; (4), acquiring biological characteristics of different types of amplified adipose mesenchymal stem cells. The amplification culture method has the advantages that high-purity CD54 (+) adipose mesenchymal stem cell population can be obtained by the aid of the amplification culture method and is high in clone formation ability, self-renewal ability and adipogenic differentiation potential, novel methods can be provided for acquiring large quantities of stem seed cells in an in-vitro manner and applying the stem seed cells to regenerating adipose tissues, a novel treatment strategy can be provided for improving clinical repair for injury of soft tissue or congenital defects and abnormality of soft tissues of organs on body surfaces, and sufficient theoretical bases can be provided for guaranteeing the safety of clinical application of the adipose mesenchymal stem cells in the future.
Owner:黎洪棉
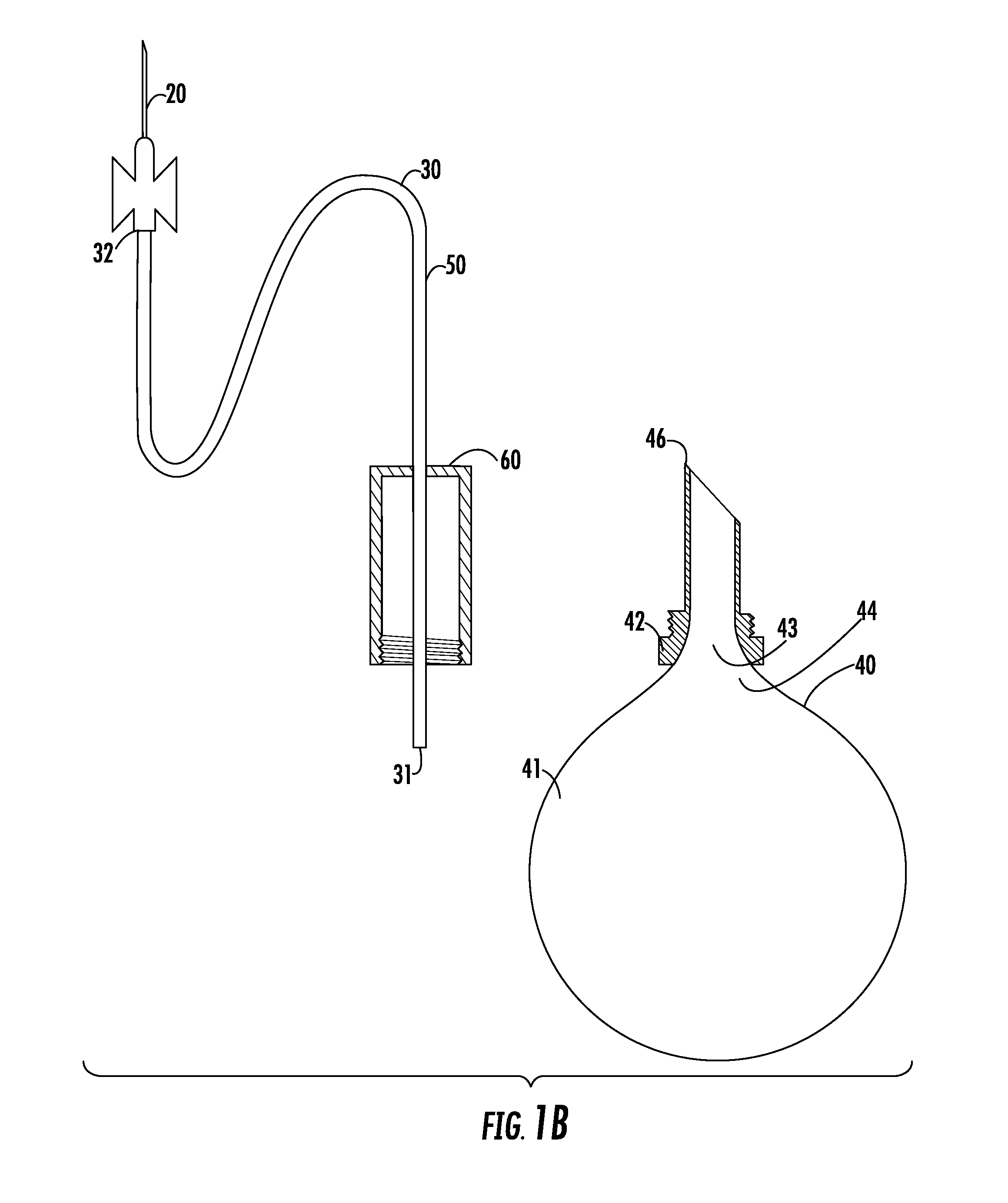
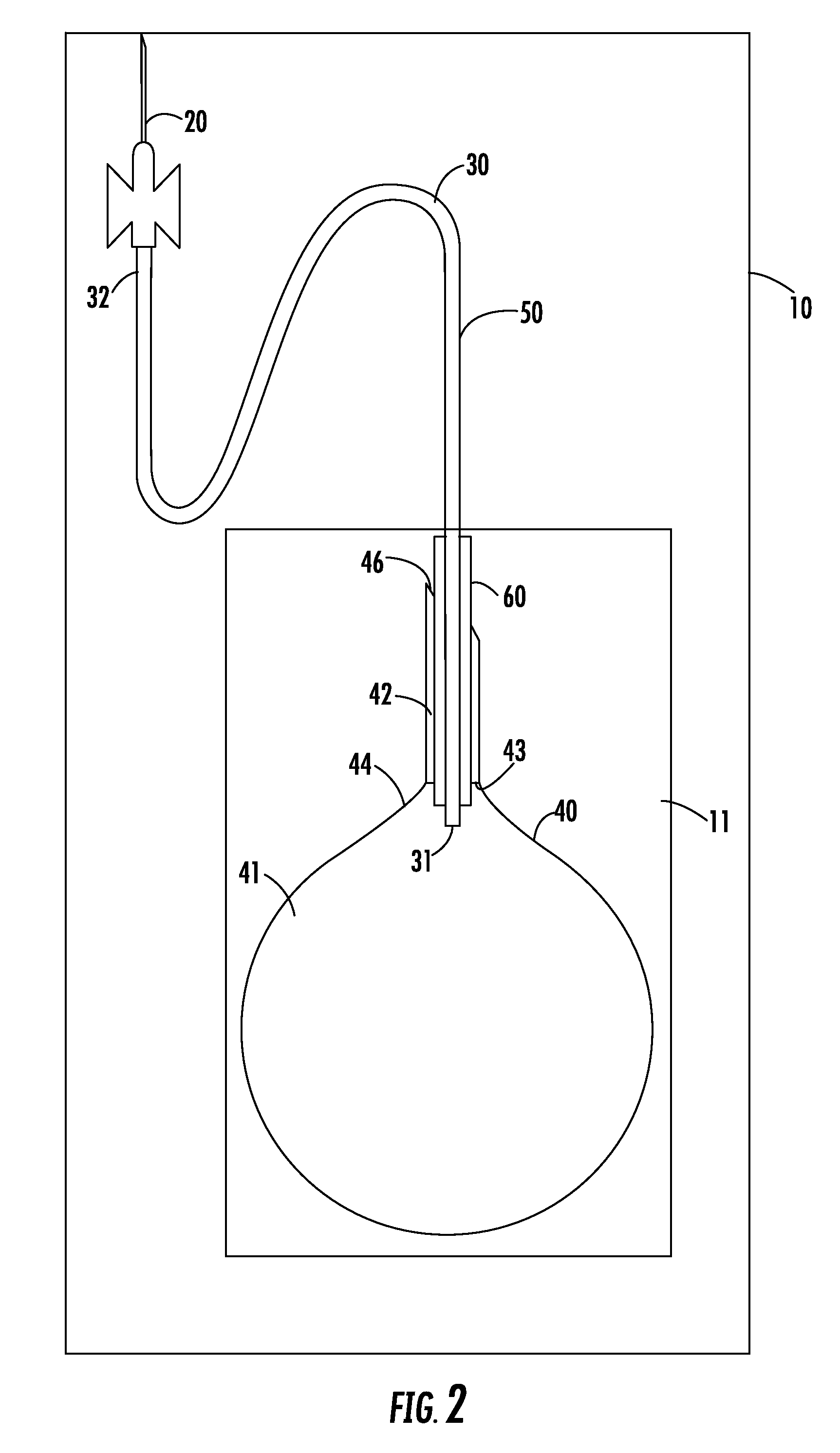
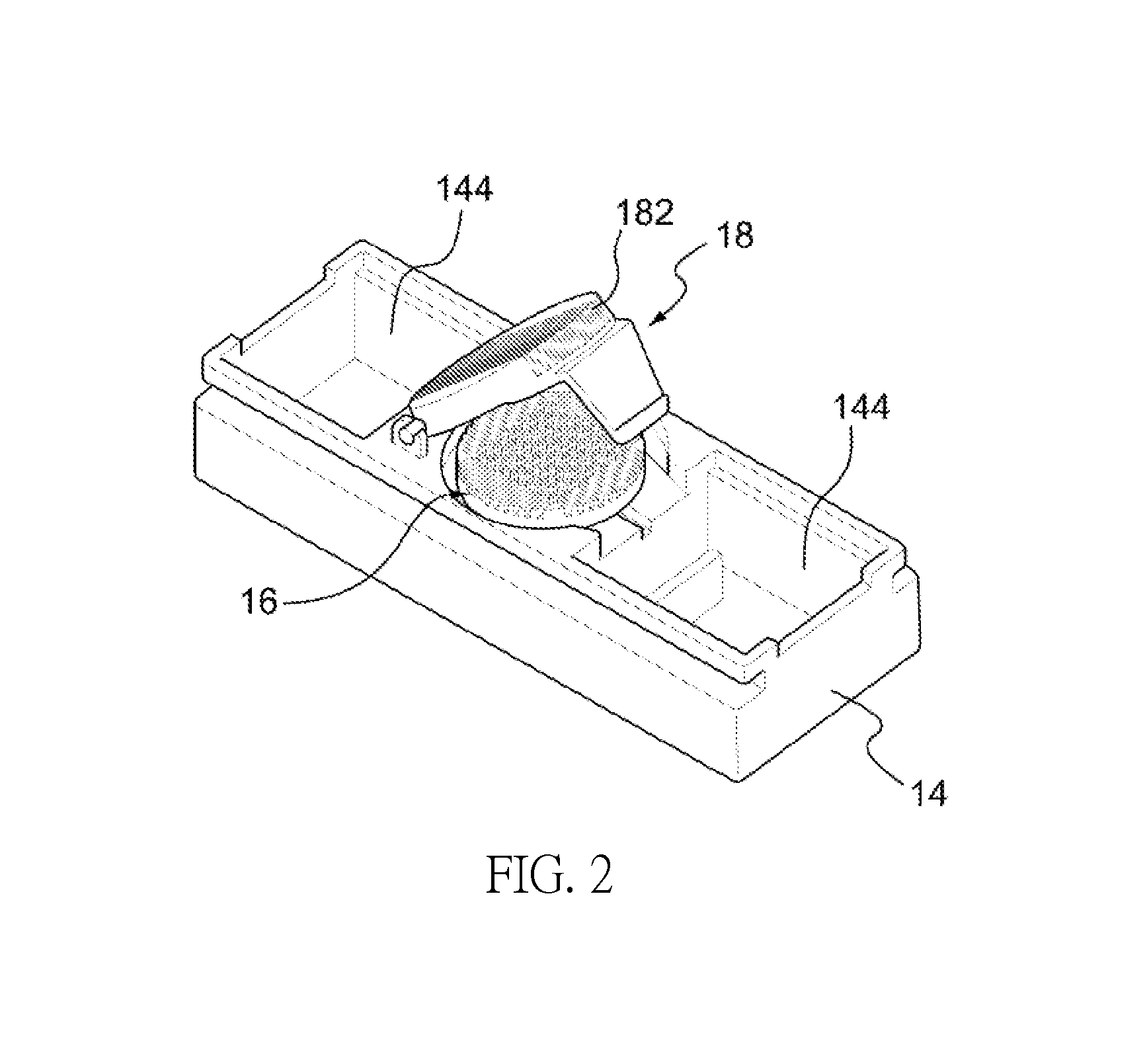
Apparatus and method for breast reconstruction and augmentation using an autologous platelet-rich fibrin matrix
InactiveUS20150148901A1Less invasive procedurePrevent coagulationBiocideMammary implantsBlood collectionAutologous platelet
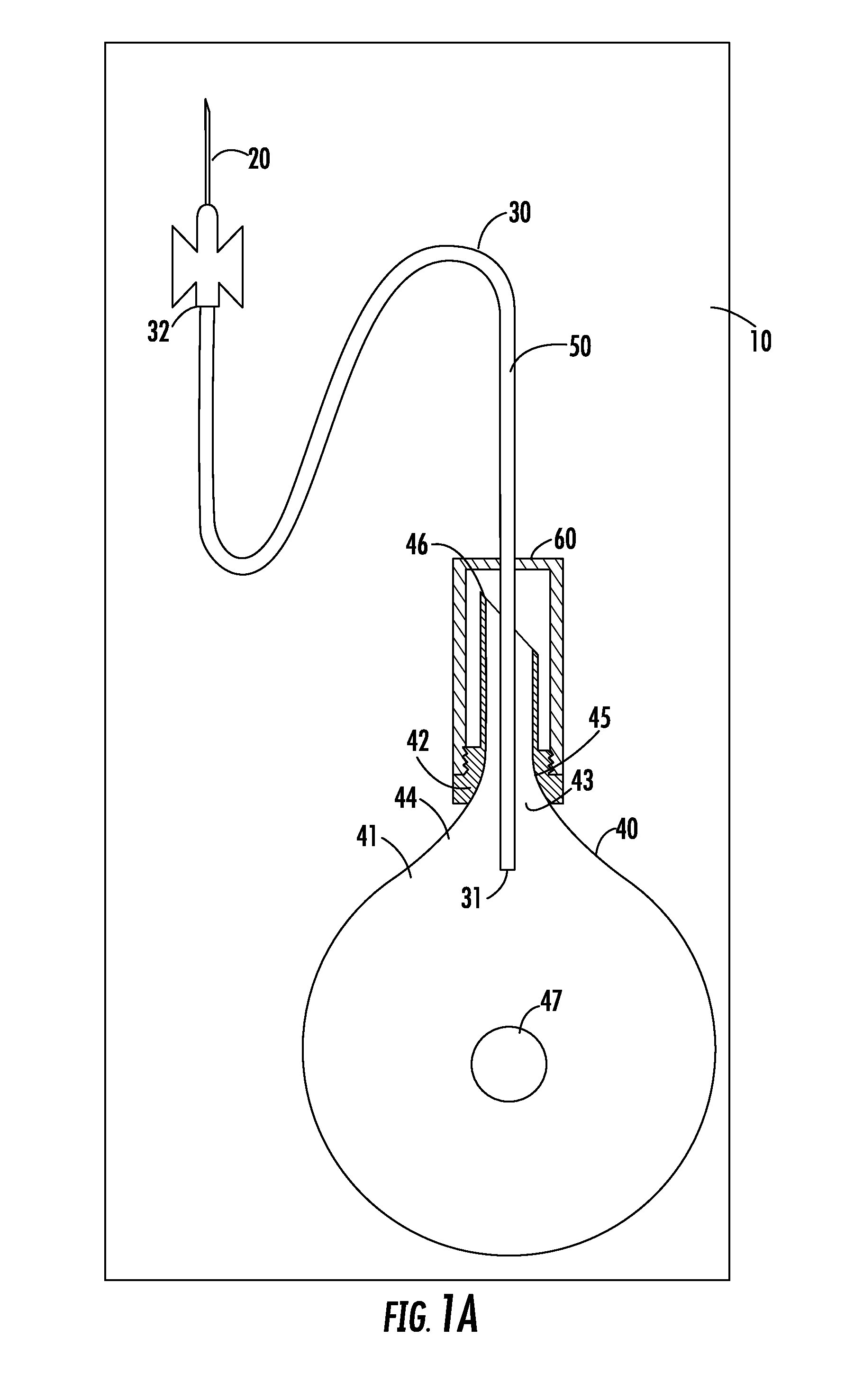
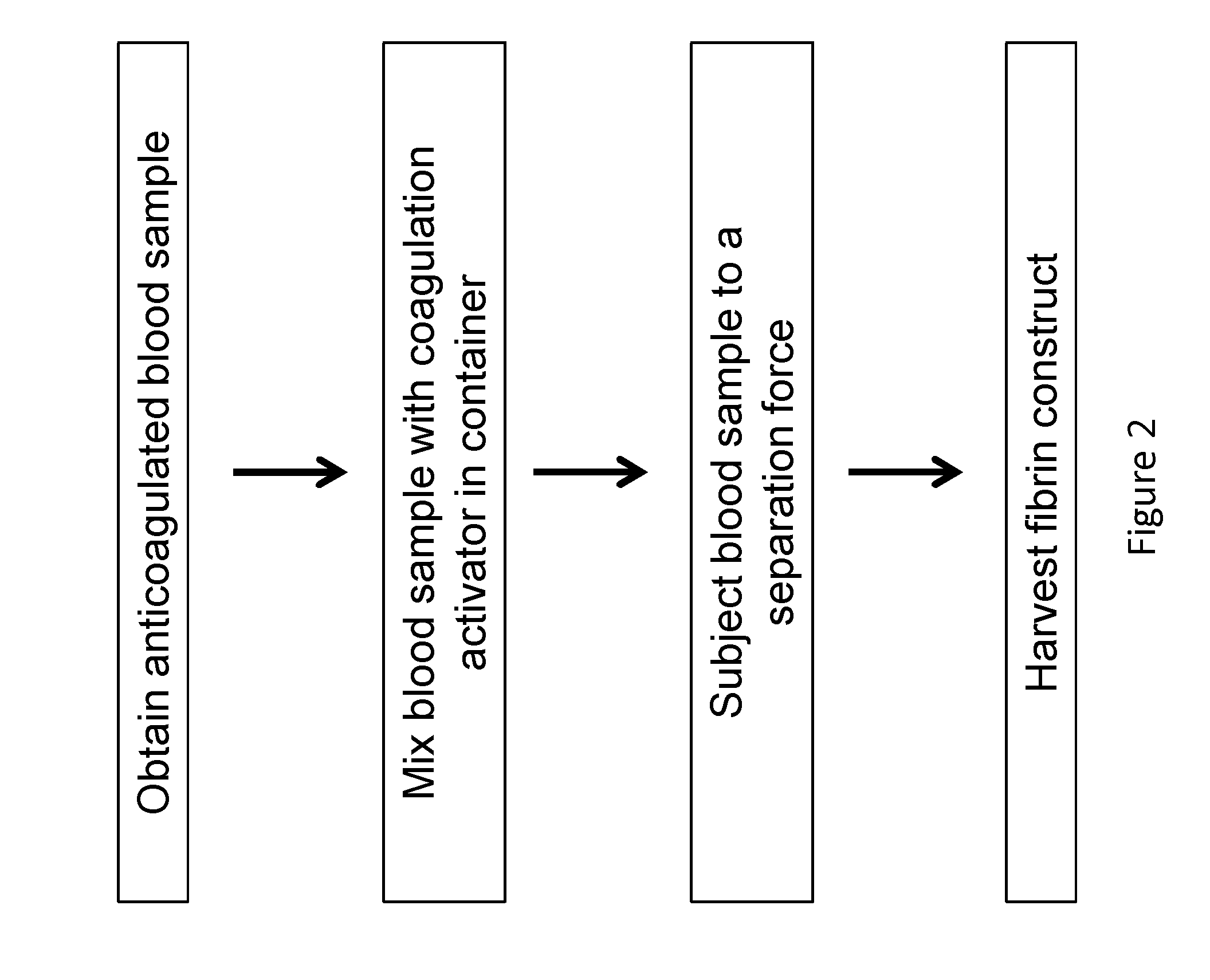
Described herein are systems and methods for soft tissue reconstruction and augmentation using a platelet-rich fibrin matrix. In one embodiment the system of the present invention includes a blood collection apparatus capable of drawing blood from a patient, a matrix preparation container configured to hold a patient's blood while the blood is separated and coagulated to form a platelet-rich fibrin matrix therein, and a matrix delivery device configured to receive the matrix preparation container therein and compress and force the platelet-rich fibrin matrix out of the matrix preparation container and into the patient. In another embodiment the invention described herein is a method in which blood is drawn from a patient and separated and coagulated to form a platelet-rich fibrin matrix. The platelet-rich fibrin matrix is implanted into a patient at the site of a tissue defect or where tissue augmentation is desired.
Owner:NAPOLITANO ANTHONY PATRICK
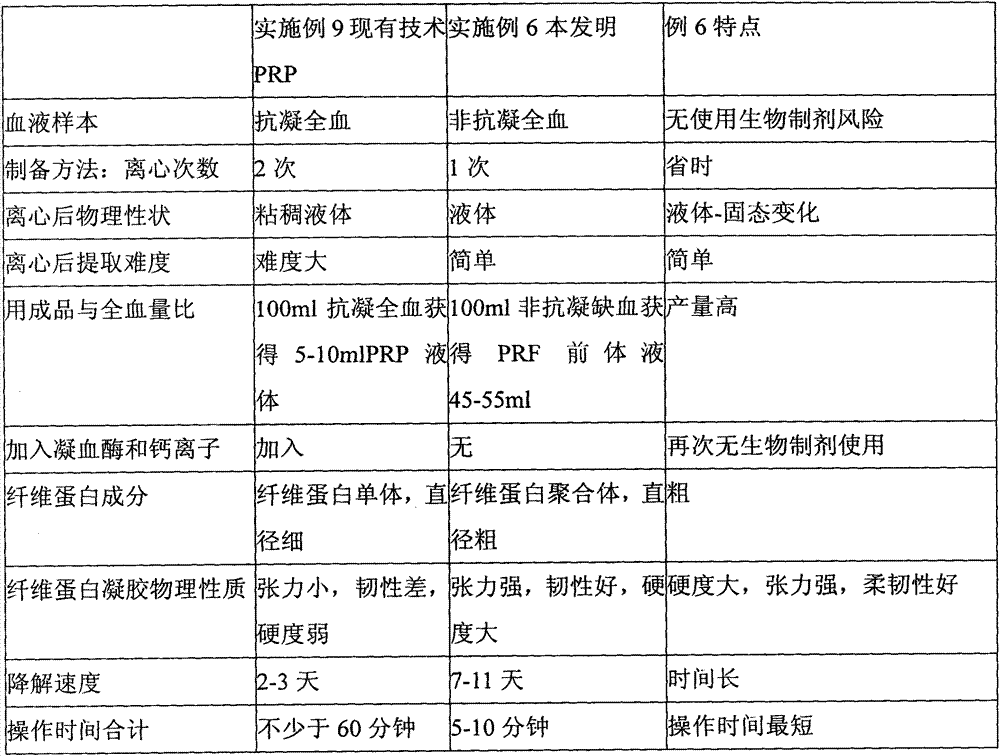
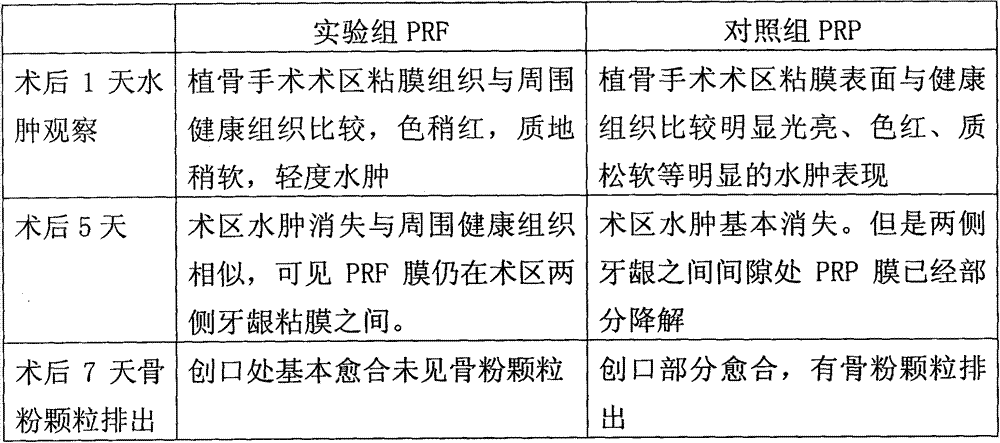
Implant material with PRF precursor liquid solidified into gel mask
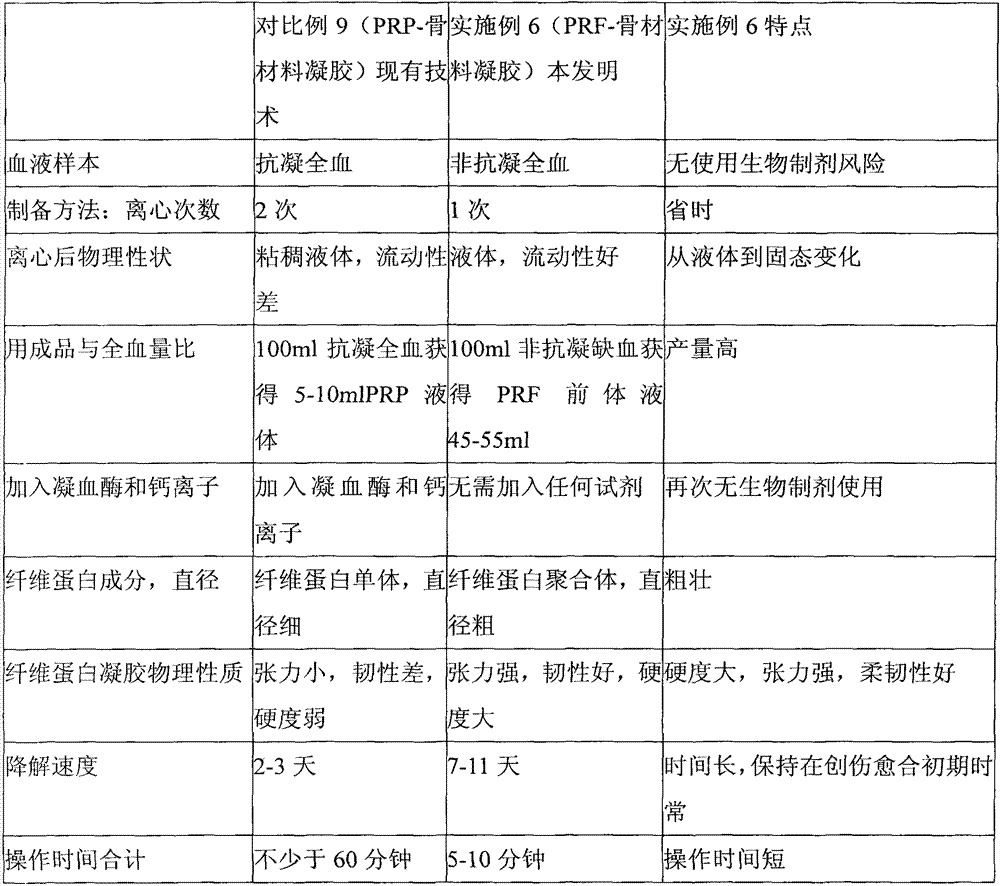
The invention relates to an implant material with a PRF (Platelet-Rich Fibrin) precursor liquid solidified into a gel mask, and belongs to the field of the implant materials for mucocutaneous operations. The implant material provided by the invention is characterized in that a non-anti-coagulation blood sample is employed, and centrifuged by a centrifuge with centrifugal force ranging from 130 to 460g, the upper total yellow liquid is put into standing at the room temperature for 30-40 minutes, and then the total yellow liquid is pressed into a mask for clinical application by a metal pressing plate. The implant material provided by the invention is high in yield, and can be naturally formed into gel without adding thrombin and calcium ions; as a result, the use risk of biochemical preparations is avoided; the operation of the implant material is simple; the PRF gel product is thick in fibrous protein filaments, good in elasticity and tenacity, strong in tension, high in hardness, slow in degradation, and not easy to break by tearing; almost no damage is caused in the preparation process of hemameba, blood platelet and shed particles of the two; and after being implanted in vivo, the implant material slowly releases various growth factors and anti-inflammatory cytokines to promote wound healing.
Owner:PEKING UNIV SCHOOL OF STOMATOLOGY
Preparation method of growth-factor-platelet-rich fibrin and releasate
InactiveCN105708858AIncrease concentrationPeptide/protein ingredientsMammal material medical ingredientsHigh concentrationMedicine
The invention discloses a preparation method of a growth-factor-platelet-rich fibrin and releasate; the preparation method includes the following steps: providing mammalian autologous blood, and placing the blood in a container; shaking the blood; centrifuging the blood and then allowing to be static, making the blood separated into three layers of substances in the container, wherein the upper layer and the lower layer are liquid substances and the middle layer is condensed into a bulk substance; taking out the bulk substance, and allowing the bulk substance to stand until a liquid substance is released; and separating to take the liquid substances, namely the growth-factor-platelet-rich fibrin and releasate. According to the preparation method of the growth-factor-platelet-rich fibrin and releasate, through the steps of shaking the blood and standing, the prepared growth-factor-platelet-rich fibrin can has higher-concentration growth factors compared with a studied or known platelet-rich fibrin.
Owner:GWOXI STEM CELL APPL TECH CO LTD
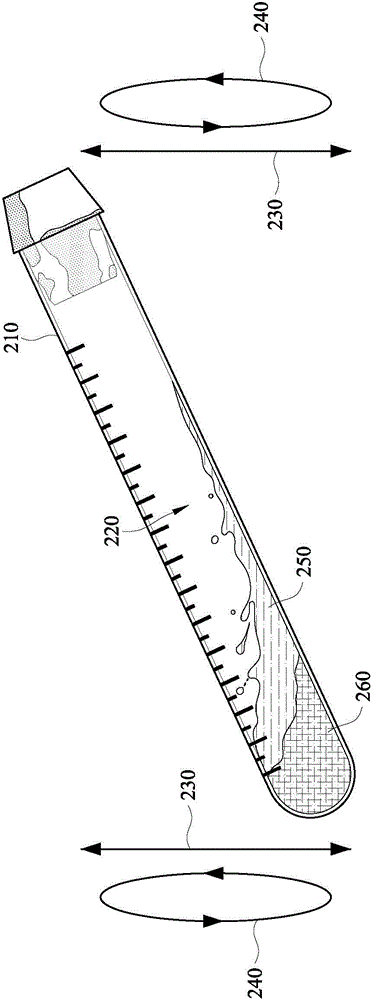
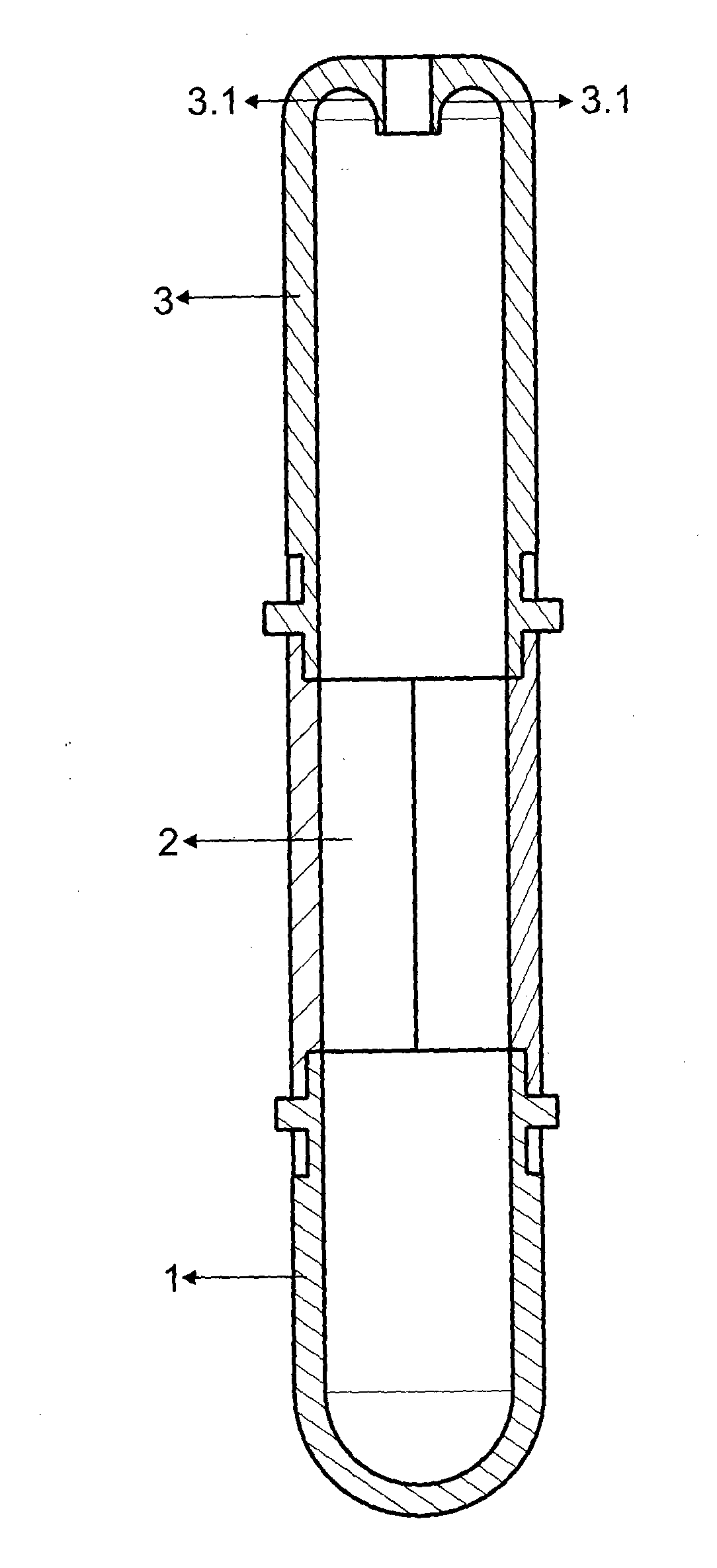
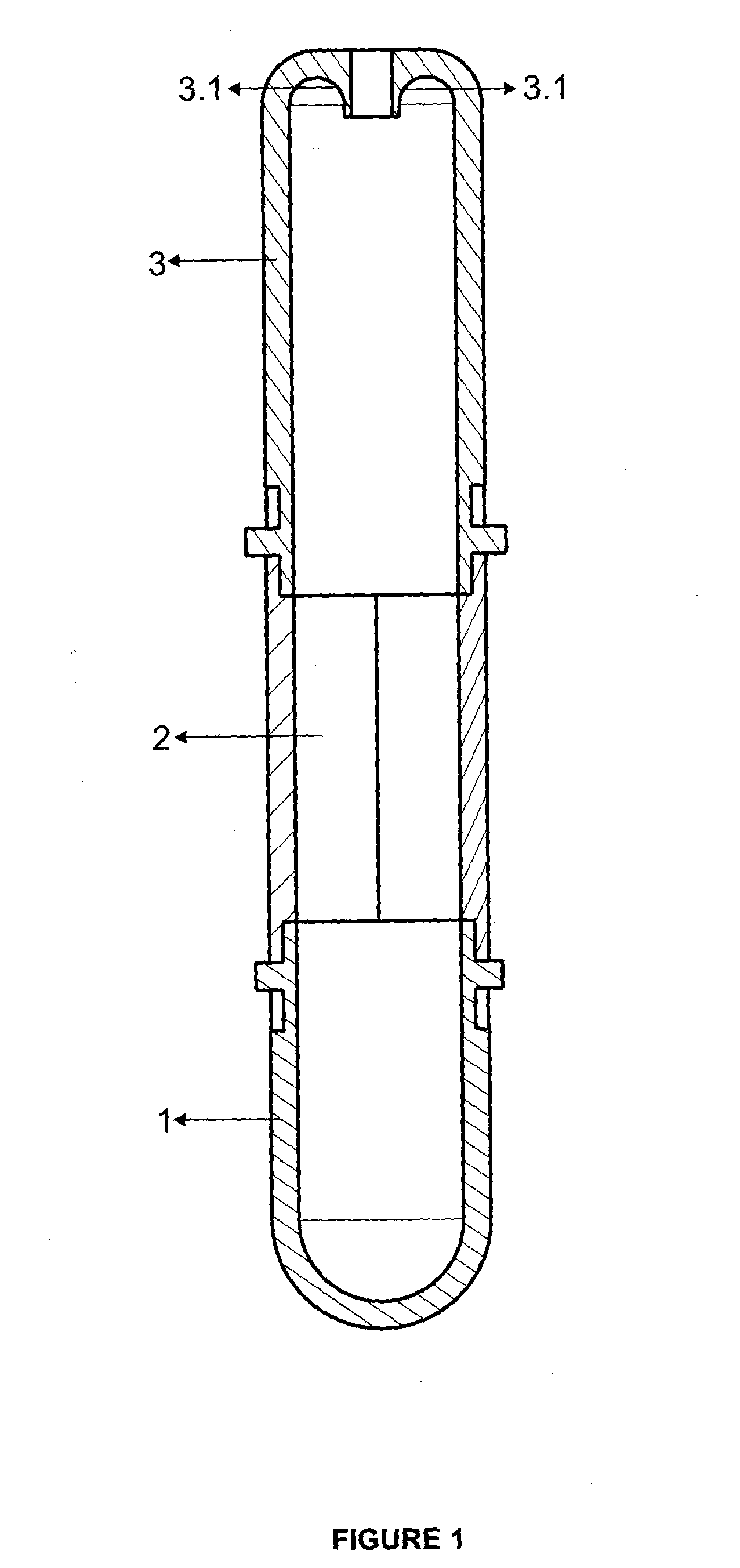
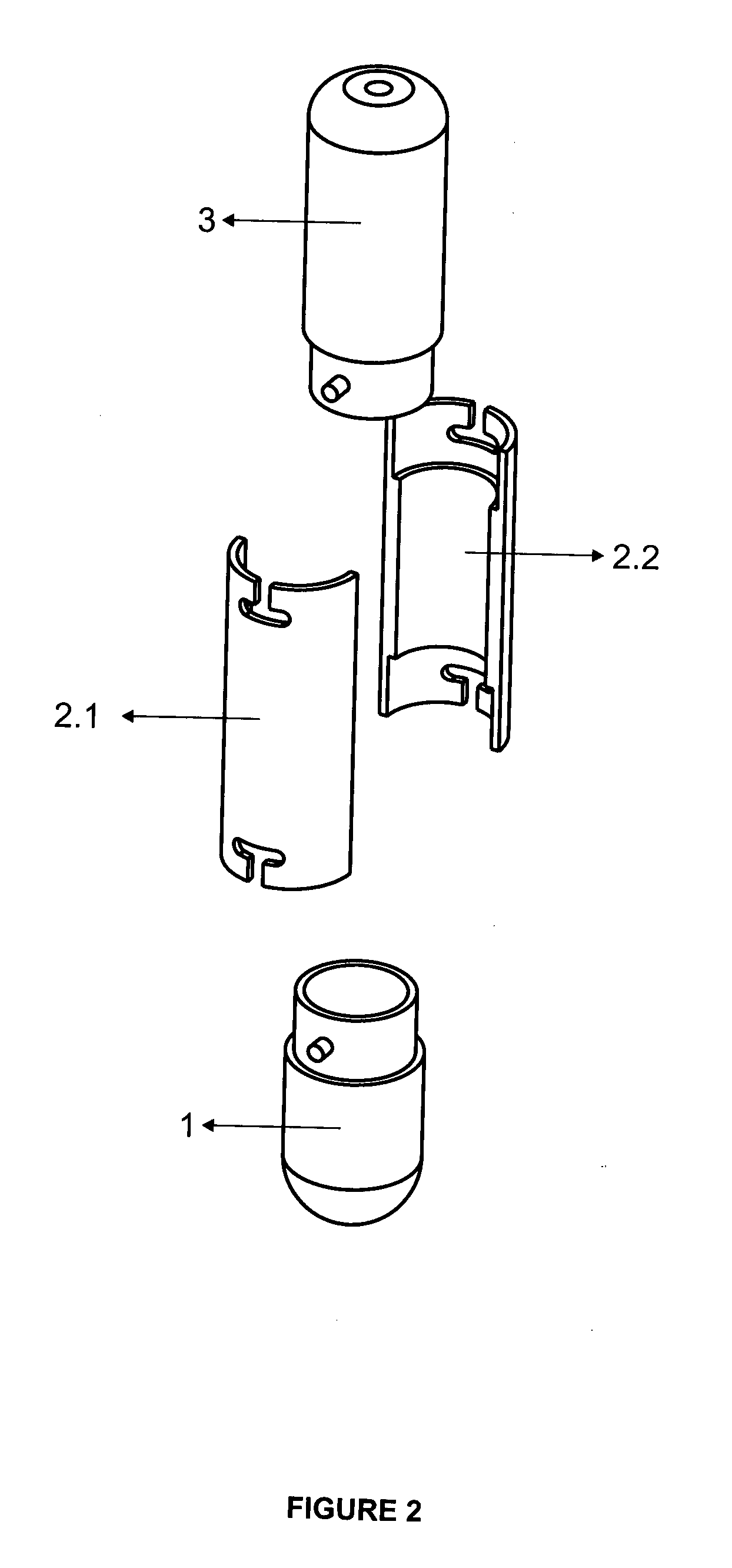
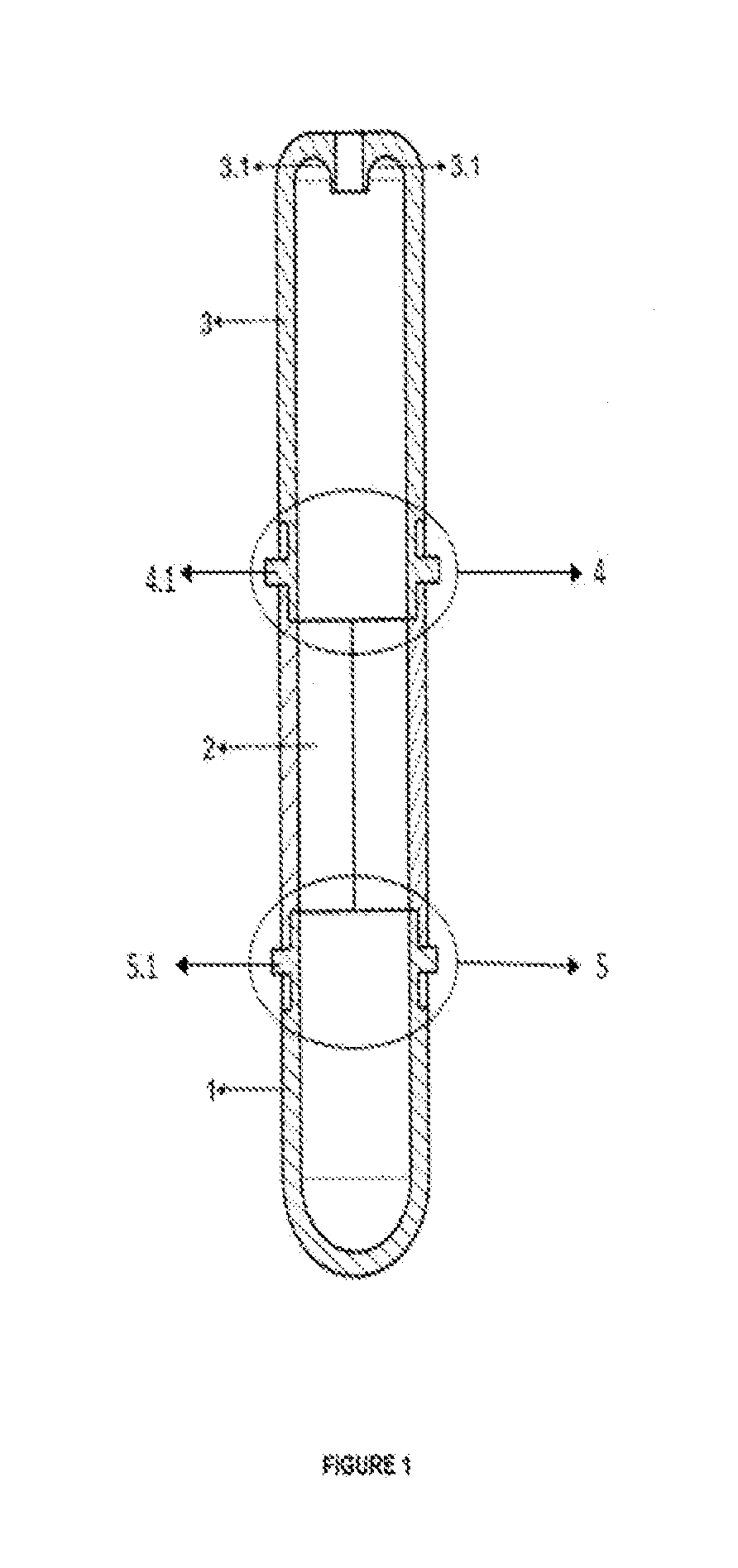
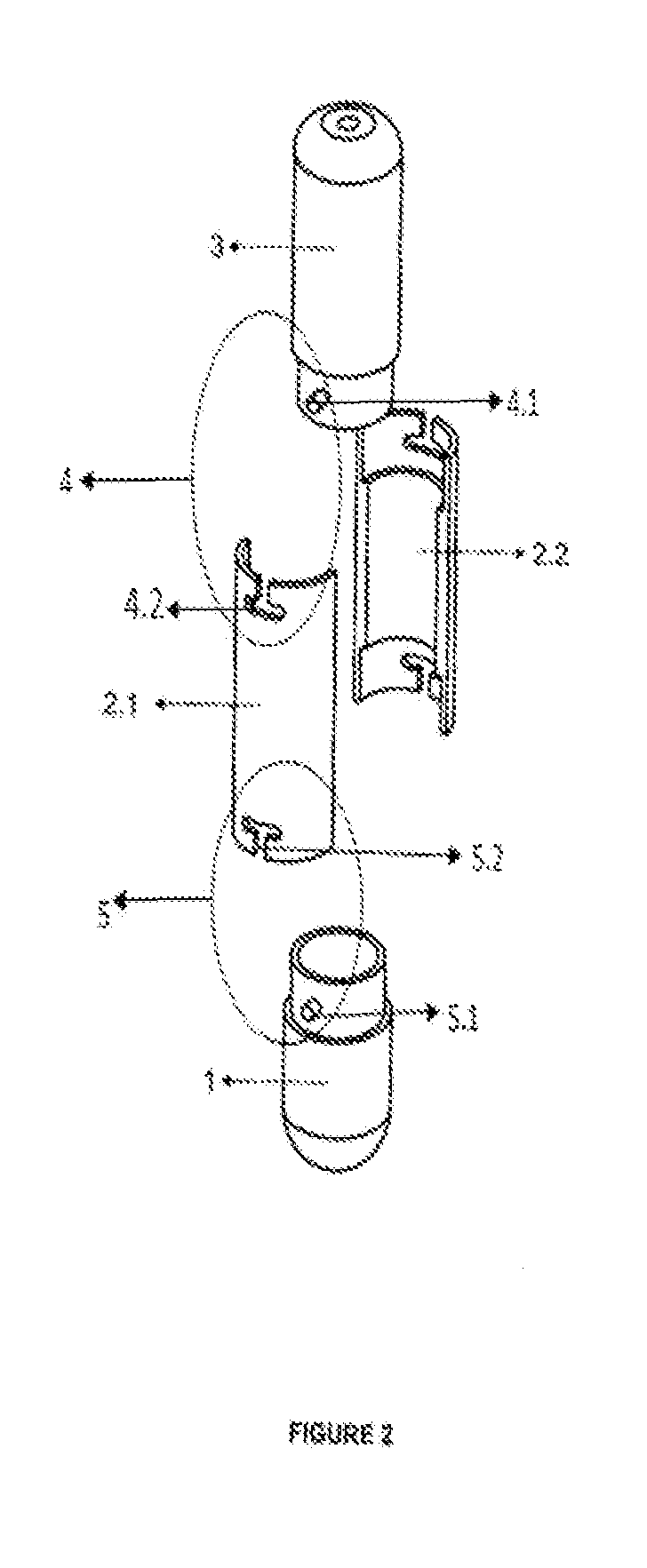
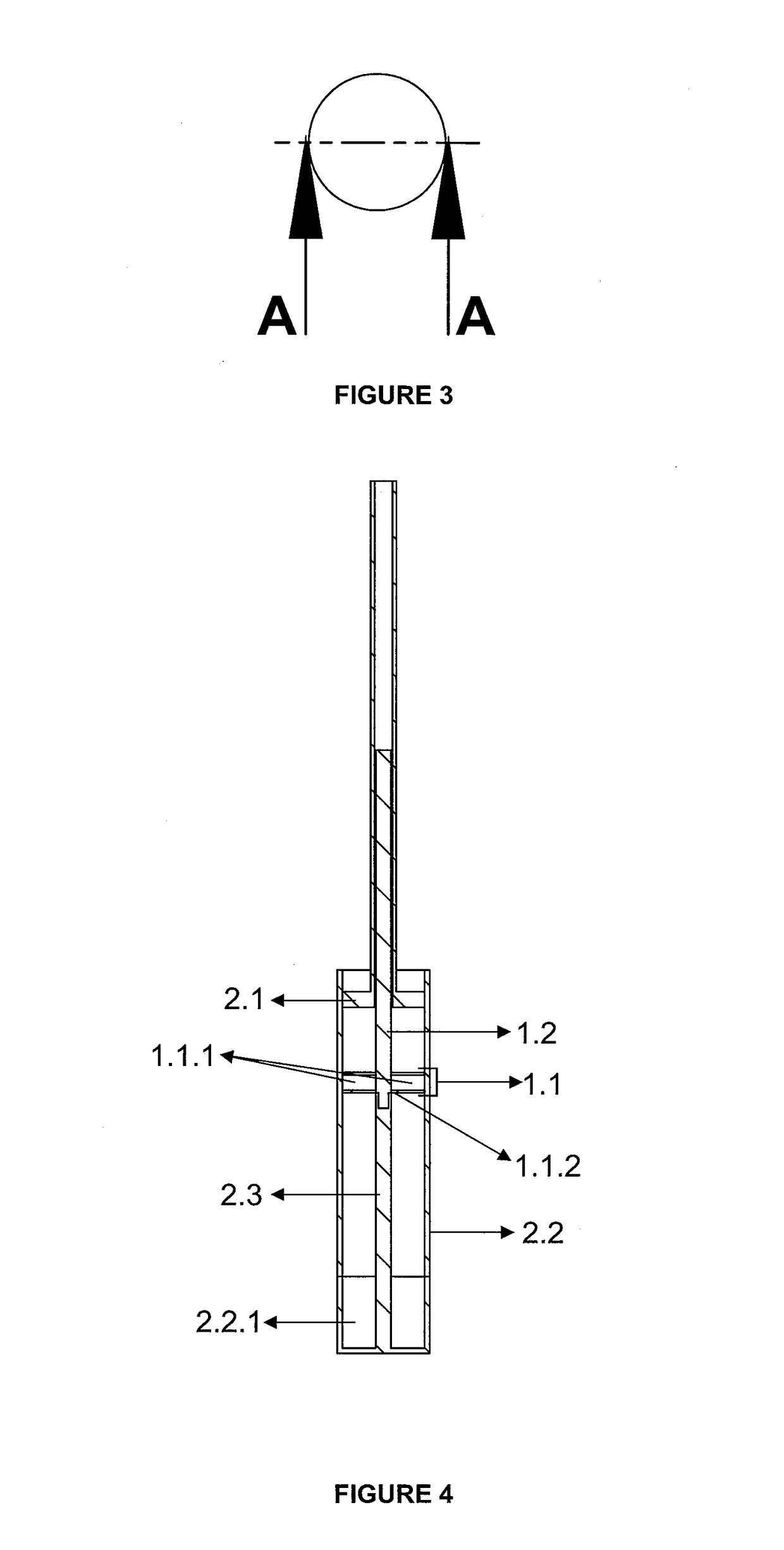
Tube to produce platelet rich fibrin
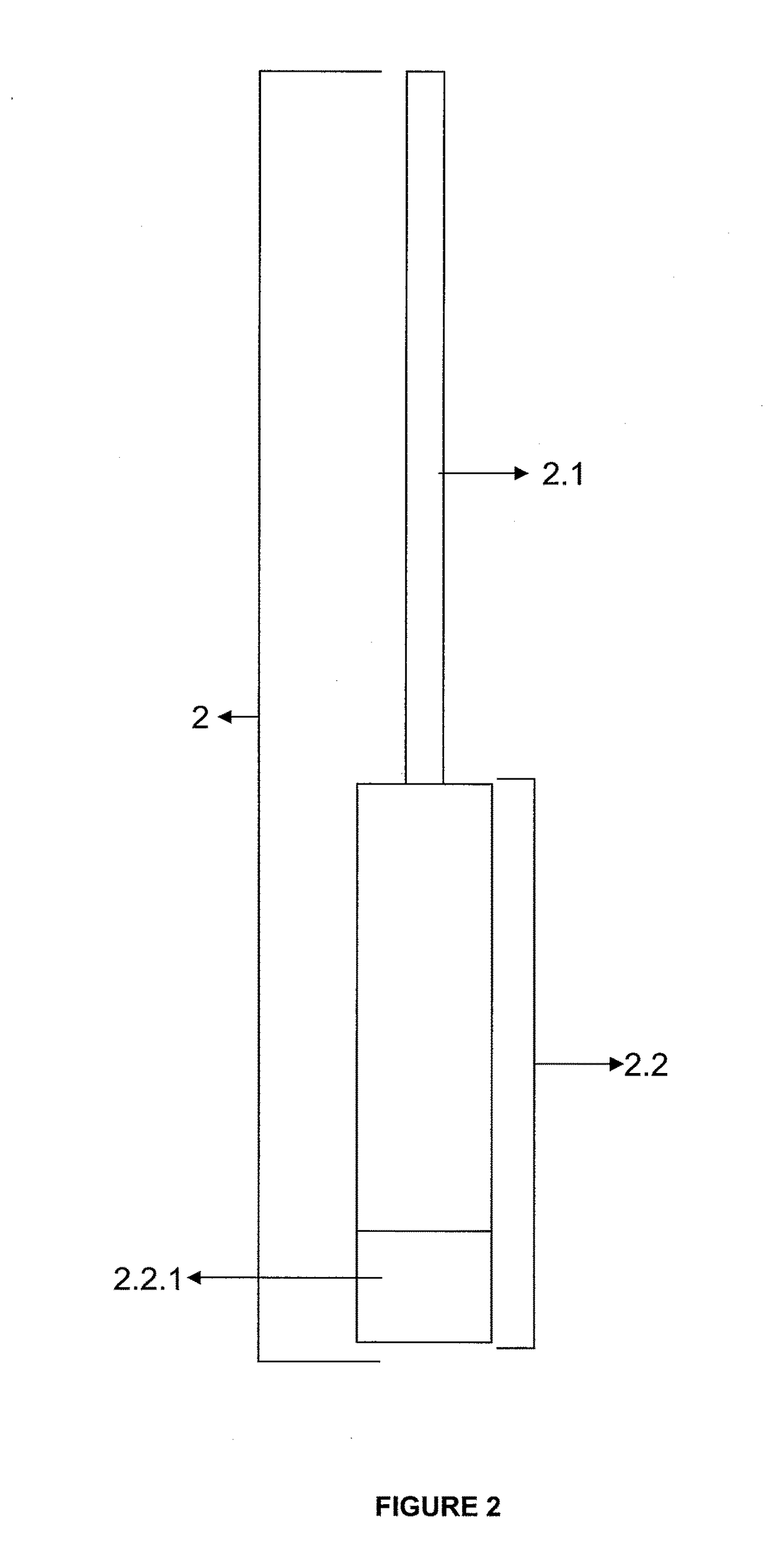
Invention; It is about the tubes used to obtain blood products through centrifuge order to cure open and closed injuries, to heal hard and soft tissues and to diagnose in all fields of medicine and dentistry. Platelet Rich Fibrin (PRF) obtained in the tubes the surface of which contacts blood and made from pure titanium or titanium alloys has a better structure of fibrin than that is obtained through classical methods. The efficiency of the high speed centrifuge is increased with this designed tube and Platelet Rich Fibrin can be removed from the tube without spoiling it.
Owner:SERHAN AKMAN +1
Composition for Accelerating Nerve Repair
InactiveUS20110318299A1Shorten recovery timeGenerate new nervesBiocideNervous disorderPlateletAnesthesia
The present invention discloses a composition for accelerating nerve repair. The composition comprises platelet-rich fibrin, a growth factor and a cytokine. The composition may further comprise a tissue with a stem cell or a neural tube to be a scaffold for promoting nerve generation. The platelet-rich fibrin is obtained from the blood of the mammal to be operated on with a nerve repair.
Owner:TAIPEI MEDICAL UNIV
Degradable tissue regeneration barrier membrane and preparing method thereof
ActiveCN109701089AFacilitate cross-linkingImprove mechanical propertiesSurgeryBarrier membraneBiological materials
The invention belongs to the field of biological materials and particularly relates to a degradable tissue regeneration barrier membrane and a preparing method thereof. The barrier membrane is prepared from, by mass, 35-55% of a calcium alginate solution, 30-40% of a chitosan solution and 5-35% of a platelet-rich fibrin solution. The barrier membrane has good mechanical performance and can effectively diminish inflammation. In addition, compared with a single-component chitosan solution, the barrier membrane has greatly reduced degradation time and meets a requirement that mechanical performance, sterilization and inflammation diminishing performance and degradation performance are integrated.
Owner:GUANGZHOU RAINHOME PHARM&TECH CO LTD
A bone graft material embedded in bone material gel in prf
Owner:PEKING UNIV SCHOOL OF STOMATOLOGY
Biotransplant for treating gum recession and restoring gingival tissue volume
Provided is a method for making a biotransplant, comprising introducing autologous fibroblasts isolated from an oral mucosa of a patient into a platelet-rich fibrin (PRF) membrane using linear retrograde needle injection, where a needle is inserted into a thickness of the PRF membrane and a first puncture is made and then a series of punctures are made that are linearly aligned or arrayed with the first puncture and are spaced at a predetermined distance from a prior puncture. A method of treatment of a periodontal tissue and a biotransplant comprising a PRF membrane and autologous fibroblasts are also provided.
Owner:OBSHCHESTVO S OGRANICHENNOJ OTVETSTVENNOSTYU VITACEL DIRECT ENGLISH TRANSLATION LLC VITACEL
Three Dimensional Healing Kit
ActiveUS20180055912A1Easy to separateGood effectDental implantsHeavy metal active ingredientsPlateletBiomedical engineering
Invention; is about the kit that enables three-dimensional healing with the platelet-rich fibrin framework support used in hard and soft tissue healing.
Owner:AKMAN SERHAN +1
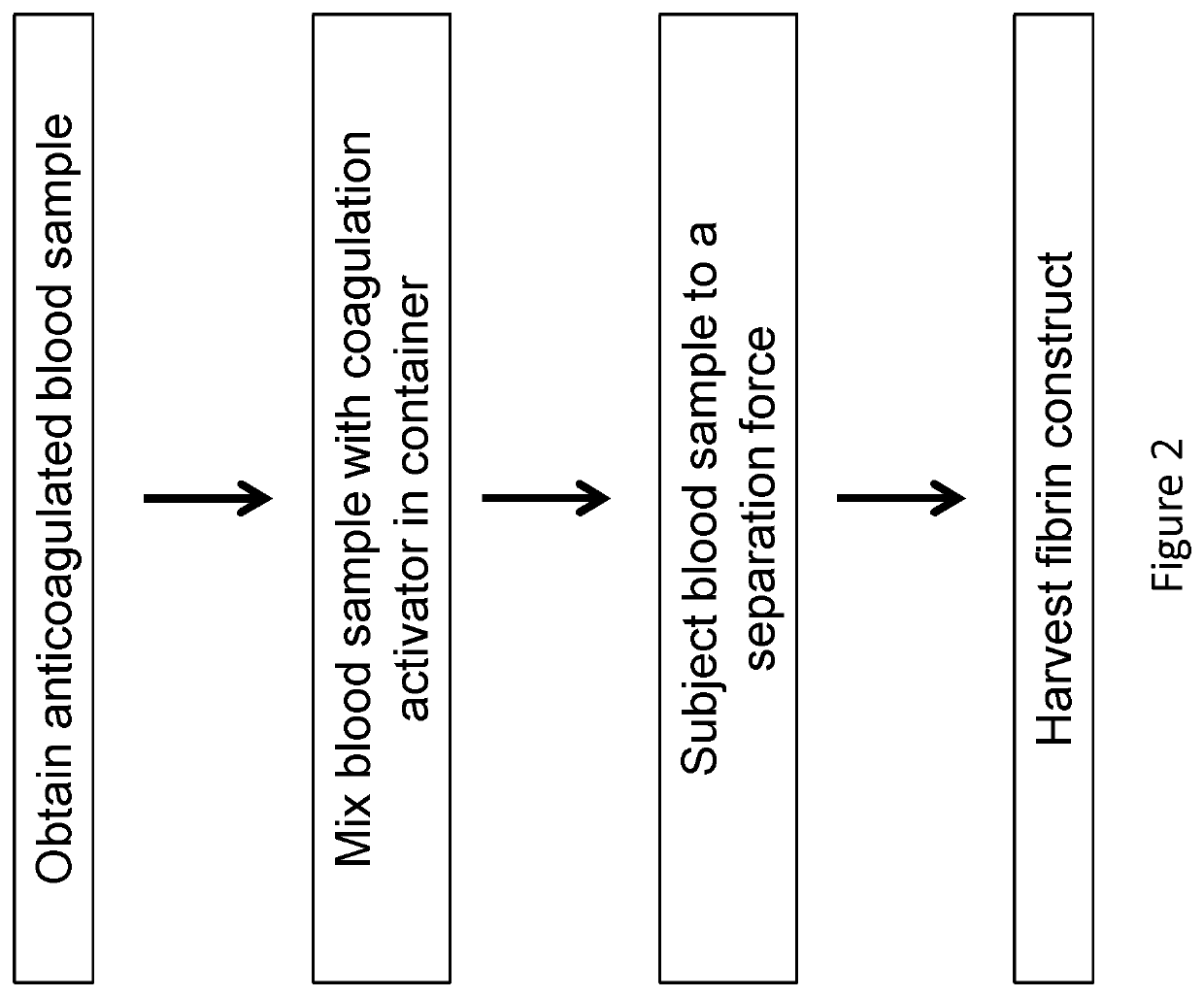
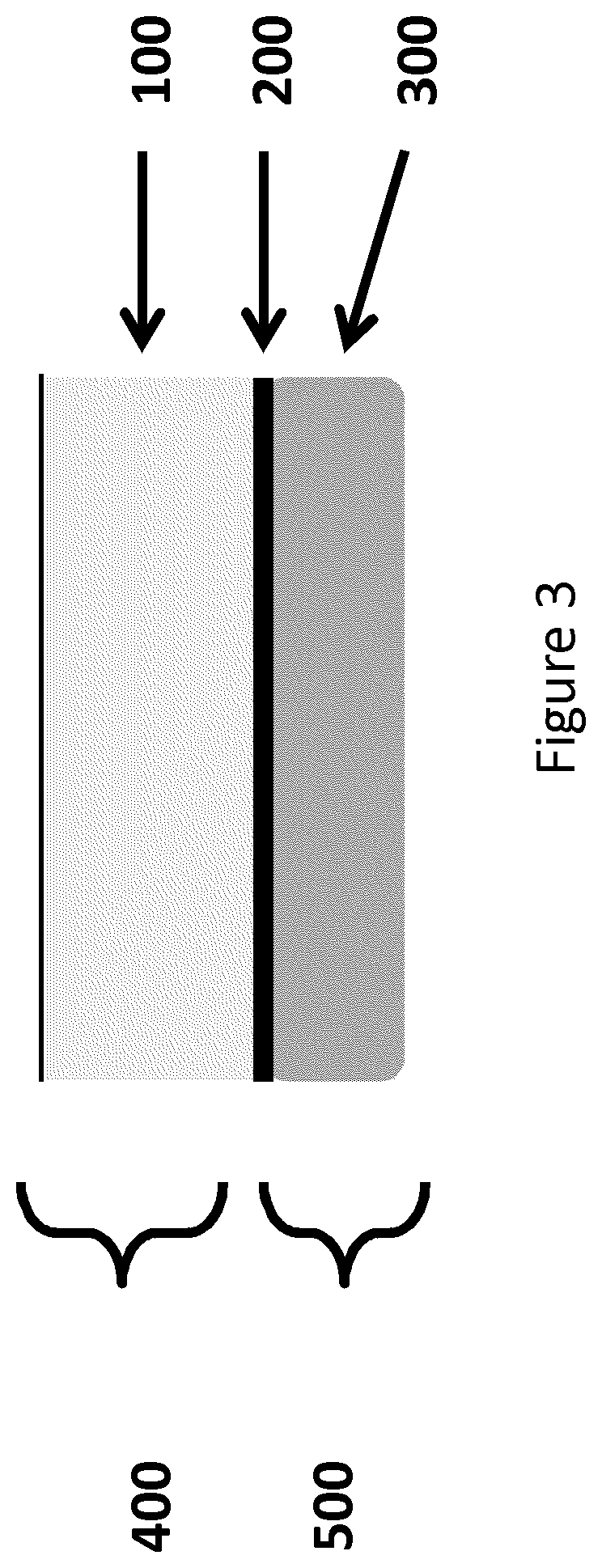
Compositions and methods for platelet enriched fibrin constructs
Compositions and methods are provided for tissue constructs that promote wound healing. The composition comprises a dimensionally stable fibrin construct for local administration to a wound site or region. In one embodiment, the fibrin construct is a wound healing composition, including components that promote wound healing, such as platelets, growth factors, white blood cells and fibrin clots. In another embodiment, the tissue treatment composition includes (i) aggregated fibrin, (ii) blood cells, and (iii) optionally, growth factors and / or other proteins.
Owner:DEPUY SYNTHES PROD INC
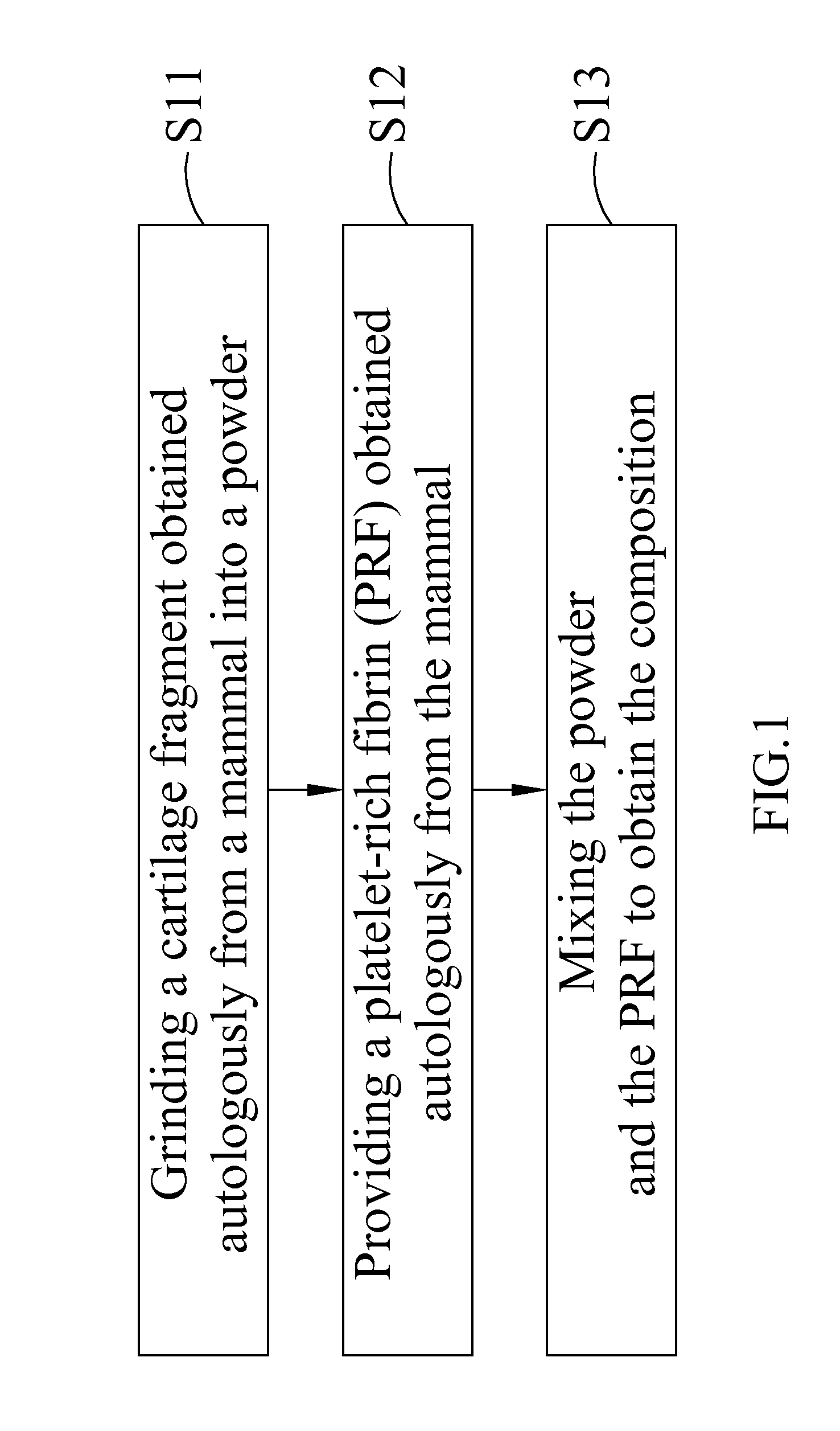
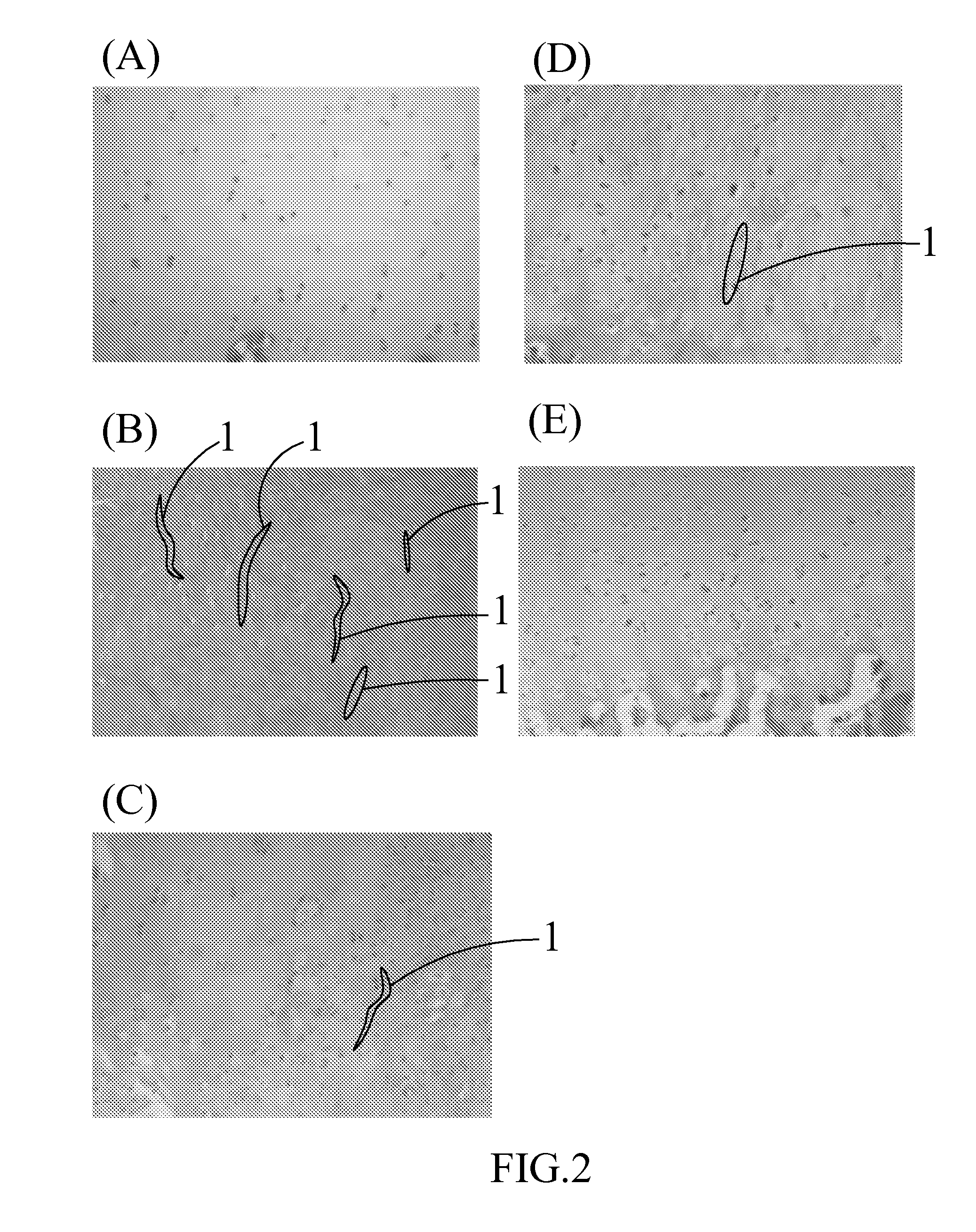
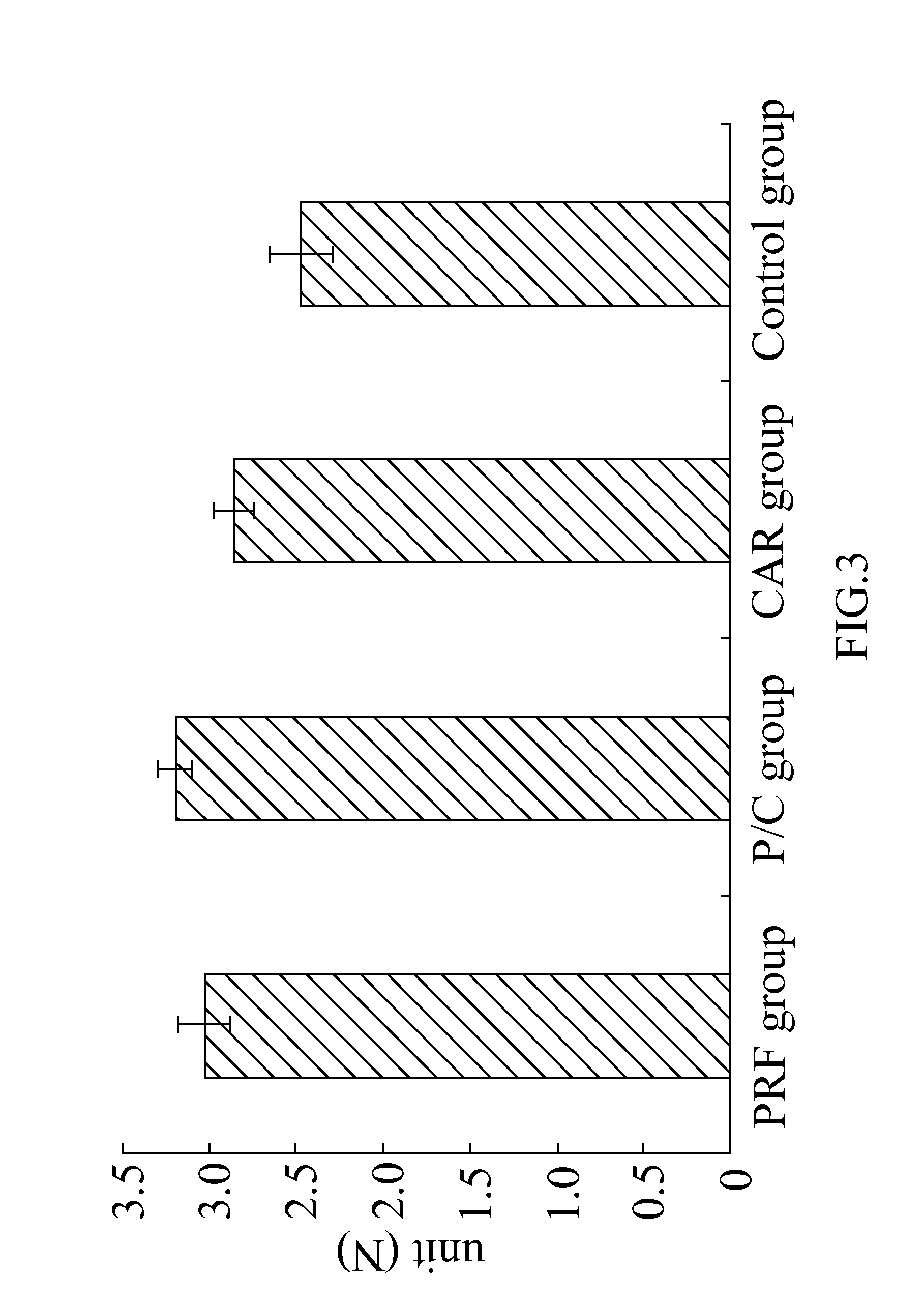
Composition for treating articular cartilage defect, and method of manufacture thereof
InactiveUS20110142793A1Promote growth factorAccelerate blood vessel regenerationBiocidePeptide/protein ingredientsPlateletSacroiliac joint
The present invention provides a composition for treating an articular cartilage defect and a manufacturing method thereof. The composition comprises a cartilage fragment and platelet-rich fibrin (PRF), and is transplanted to a surface of the defective cartilage of a mammal in order to treat the articular cartilage defect. Wherein the cartilage fragment is acquired from the cartilage of the same mammal, and the PRF is acquired from the blood of the same mammal.
Owner:TAIPEI MEDICAL UNIV
Pharmaceutical composition for treating acute kidney injury
InactiveCN108273063APeptide/protein ingredientsPharmaceutical delivery mechanismInsulin-like growth factorInduced pluripotent stem cell
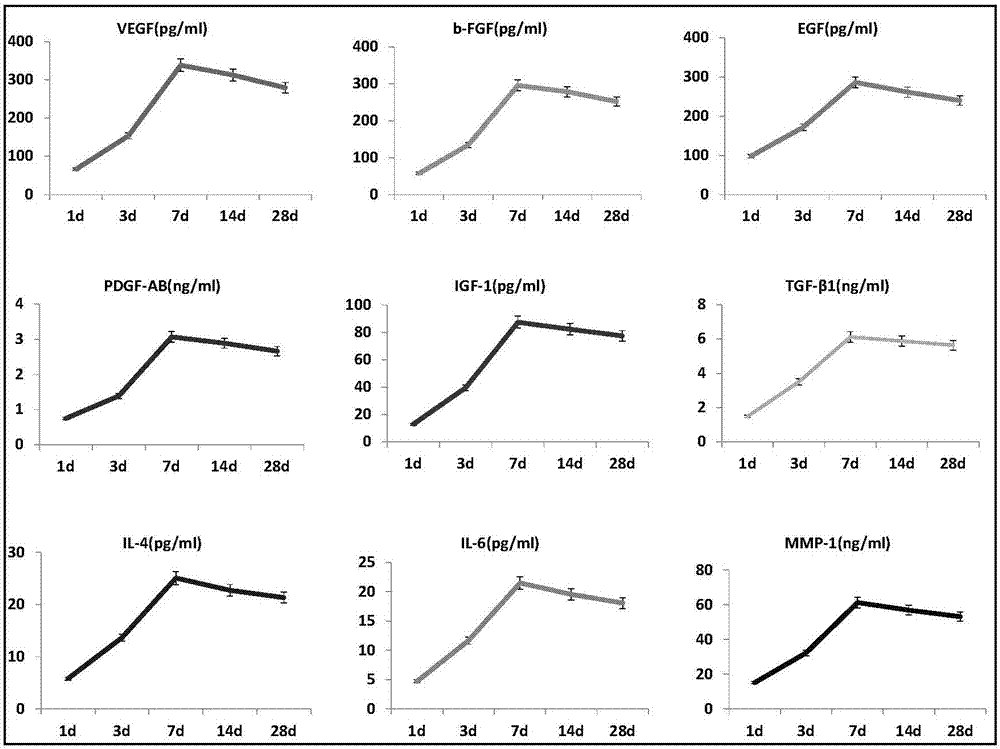
The present invention relates to a pharmaceutical composition for treating acute kidney injury, wherein the pharmaceutical composition comprises stem cells and a platelet-rich fibrin (PRF) release liquid, the stem cells are embryonic stem cells, adult stem cells or induced pluripotent stem cells, and the platelet-rich fibrin release liquid at least contains a growth factor selected from Transforming growth factor-beta1 (TGF-[beta]1), Vascular endothelial growth factor (VEGF), Platelet-derived growth factor (PDGF), Bone morphogenetic protein (BMP), Platelet factor 4 (PF4), Interleukin (IL), Epidermal growth factor (EGF), Fibroblast growth factor (FGF), Nerve growth factor (NGF), and Insulin-like growth factor (IGF).
Owner:GWOXI STEM CELL APPL TECH CO LTD
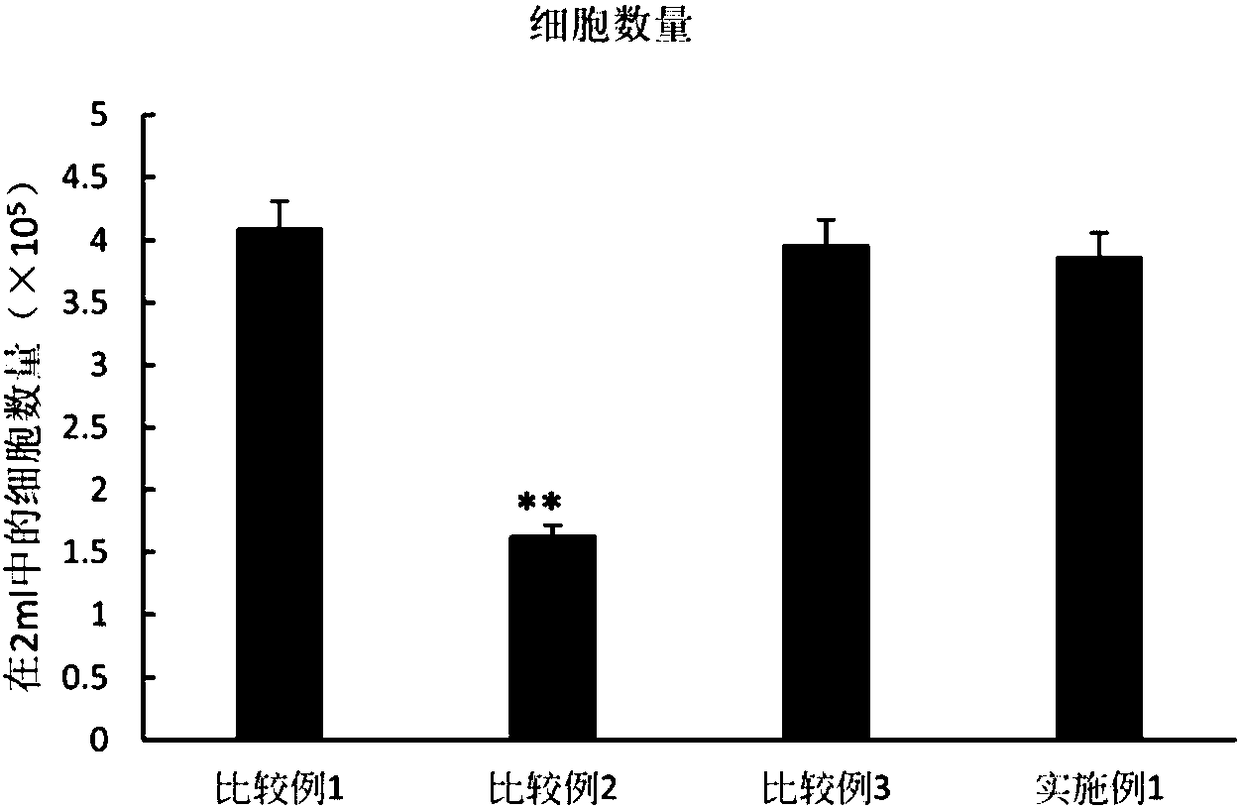
Serum fraction of platelet-rich fibrin as a cell culture additive
InactiveUS20190167723A1High proliferation rateEnhance cell viabilityPeptide/protein ingredientsPharmaceutical delivery mechanismDiseasePlatelet release
A method of preparing an isolated serum fraction of platelet rich fibrin, cell cultures comprising said serum fraction and its use as a cell culture additive. The invention also relates to increasing proliferation rate of chondrocytes, to the treatment of articular or joint diseases and to increasing the proliferation rate of mesenchymal stem cells. The isolated serum fraction of platelet rich fibrin (PRF), is prepared by providing platelet rich plasma (PRP) without the addition of an anticoagulant; clotting the PRP to obtain a coagel of PRF; and separating the coagel to isolate the serum fraction which comprises an activated platelet releasate; and further provides for the isolated serum fraction obtained by such method, and its medical use.
Owner:LACERTA TECH
Serum fraction of platelet-rich fibrin
ActiveUS9480716B2Efficient activationMaintaining the integrity of platelet structuresBiocideInanimate material medical ingredientsAnticoagulantPlatelet release
The invention provides for a method of preparing an isolated serum fraction of platelet rich fibrin (PRF), comprising the steps ofa. providing platelet rich plasma (PRP) without the addition of an anticoagulant;b. clotting the PRP to obtain a coagel of PRF; andc. separating the coagel to isolate the serum fraction which comprises an activated platelet releasate;and further provides for the isolated serum fraction obtained by such method, and its medical use.
Owner:LACERTA TECH
Bone grafting material by embedding bone material gel in PRF (platelet-rich fibrin)
The invention relates to a bone grafting material by embedding a bone material gel in PRF (platelet-rich fibrin), and belongs to the field of bone grafting operation. The bone grafting material is prepared by the following steps: performing centrifugal operation on a non-anticoagulant blood sample by a centrifugal machine at a centrifugal force of 130-460g; mixing all upper yellow liquid and the bone material, and standing for 30-40 minutes at room temperature to obtain the bone grafting material. The bone grafting material is a whole clot, PRF in the product reacts with the bone material, leukocyte and platelet in PRF are uniformly distributed, and PRF is widely and comprehensively contacted with the bone material. The bone grafting material is high in plasticization, and is a perfect bone grafting material with a complete frame supporting structure.
Owner:PEKING UNIV SCHOOL OF STOMATOLOGY
Tissue repair growth factor sustained-release material, preparation method and application
InactiveCN106512089APromote regenerationImprove regenerative abilityTissue regenerationProsthesisAspirinTissue repair
The invention relates to an anti-inflammatory repair growth factor sustained-release material, a preparation method and an application. The anti-inflammatory tissue repair growth factor sustained-release material consists of platelet-rich fibrin and aspirin; and the preparation method comprises the following steps: S1, preparing aspirin complex liquid; S2, collecting venous blood; and S3, mixing the aspirin complex liquid with the venous blood, and conducting centrifuging, so as to obtain a jelly substance in an intermediate layer, namely the anti-inflammatory tissue repair growth factor sustained-release material which is a compound of the platelet-rich fibrin and the aspirin. The anti-inflammatory tissue repair growth factor sustained-release material provided by the invention is mainly applied to periodontal guided tissue regeneration or bone tissue regeneration. According to the anti-inflammatory tissue repair growth factor sustained-release material and the preparation method provided by the invention, tissue regeneration can be effectively promoted, local immunity and stem cell function can be regulated, inflammatory response can be relieved and a regeneration effect can be enhanced.
Owner:BEIJING STOMATOLOGY HOSPITAL CAPITAL MEDICAL UNIV
Preparation method and use of graft material in double membrane structure
The invention belongs to the field of tissue engineering and biomaterials and discloses a preparation method and use of a graft material in a double membrane structure. The graft material comprises stem cell membrane patch segments and platelet-rich fibrin membrane particles, wherein the stem cells are firstly expanded-cultured, and then induction-cultured by using a membrane induction fluid to obtain a stem cell membrane patch, and the stem cell membrane patch is sheared to prepare the cell membrane patch segment; a platelet-rich fibrin gel is extruded, then liquid content of the extruded platelet-rich fibrin gel is removed to obtain the platelet-rich fibrin membrane, and the platelet-rich fibrin membrane is sheared to form particles; and the cell membrane patch segments and the platelet-rich fibrin membrane particles are mixed according to an optimal proportioning relation of in-vitro screening to prepare the needed graft material. The graft material can be widely applied to tissue repair and regeneration of small-area lesion in oral activity and other parts of the body, and the tissue repair effect is improved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
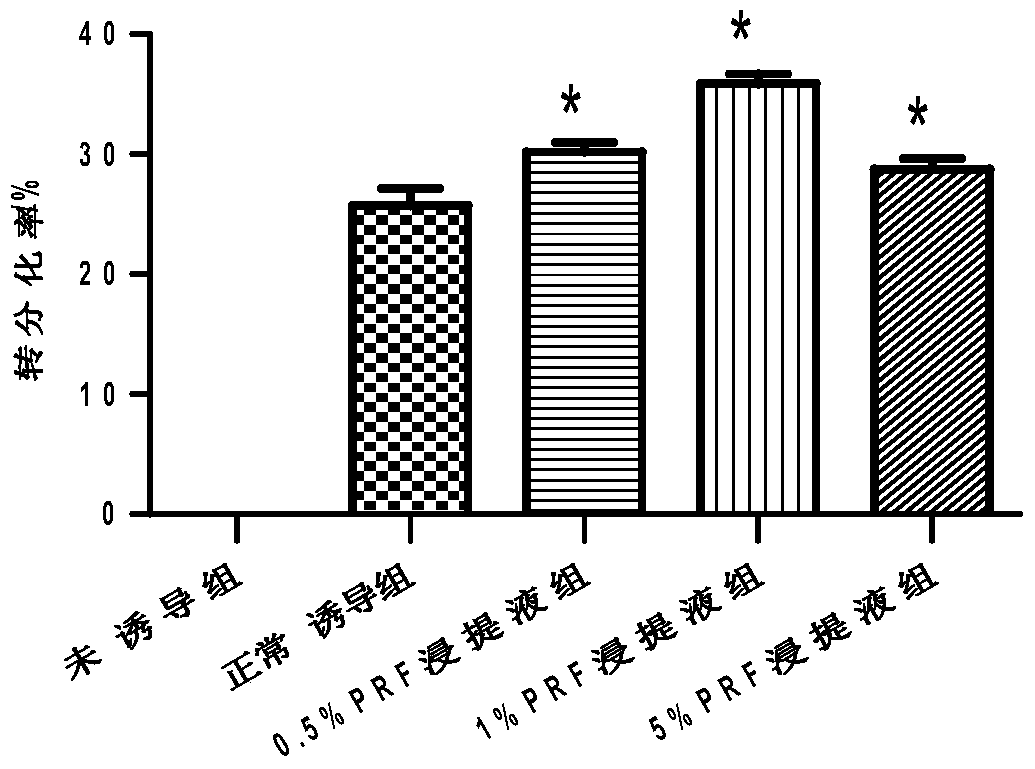
A method for improving the transdifferentiation of adipose-derived stem cells into salivary acinar-like cells
ActiveCN104928239BImprove functional statusPromotes transdifferentiationArtificial cell constructsSkeletal/connective tissue cellsDigestionSalivary gland cell
Salivary gland cells and adipose-derived stem cells are respectively cultured by adopting a digestion method; co-culture is performed on the salivary gland cells and the adipose-derived stem cells with a transwell culture chamber and a 24-well culture plate; the salivary gland cells are inoculated in an inner chamber, and the adipose-derived stem cells are inoculated in an outer chamber; a DMEM (dulbecco's modified eagle medium) containing fetal calf serum of which the volume fraction is 10 percent is adopted as the culture medium; platelet-rich fibrin leach liquor of which the mass fraction is 0.5 to 5 percent is added to induce the transdifferentiation of the adipose-derived stem cells into the salivary gland acinus-like cells. According to a method disclosed by the invention, the efficiency of the transdifferentiation of the adipose-derived stem cells into the salivary gland acinus-like cells can be effectively improved, and seed cells and a new transdifferentiation method are provided for the tissue engineering technology, regenerative medicine research and application.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
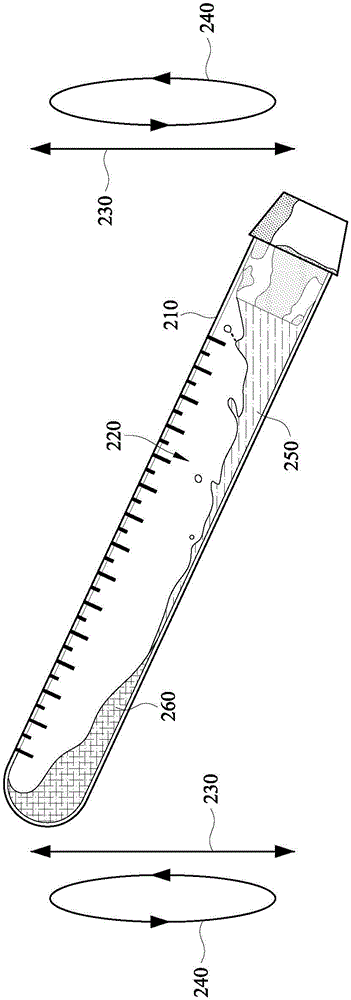
Tube to produce platelet rich fibrin
Invention; It is about the tubes used to obtain blood products through centrifuge in order to cure open and closed injuries, to heal hard and soft tissues and to diagnose in all fields of medicine and dentistry. Platelet Rich Fibrin (PRF) obtained in the tubes the surface of which contacts blood and made from pure titanium or titanium alloys has a better structure of fibrin than that is obtained through classical methods. The efficiency of the high speed centrifuge is increased with this designed tube and Platelet Rich Fibrin can be removed from the tube without spoiling it.
Owner:SERHAN AKMAN +1
Method for repair of articular cartilage defect and a device used therein
Owner:TAIPEI MEDICAL UNIV
Fibrin composition, substrate for regenerative medicine, method for manufacturing fibrin composition, and kit
PendingUS20210008248A1Promote blood coagulationPromote bone regenerationDental implantsConnective tissue peptidesBiochemistryCollagen fibril
An object of the present invention is to provide a fibrin composition manufactured by a method as a substitute for a method for manufacturing platelet-rich fibrin (PRF) triggered by the activation of a coagulation factor, a substrate for regenerative medicine using the fibrin composition, a method for manufacturing a fibrin composition, and a kit for being used in the method. According to the present invention, there is provided a fibrin composition including blocks, which each include a protein having repetitive Arg-Gly-Asp sequences derived from human collagen, fibrin, and platelets.
Owner:FUJIFILM CORP
An implant material that solidifies prf precursor fluid into a gel film
The invention relates to an implant material with a PRF (Platelet-Rich Fibrin) precursor liquid solidified into a gel mask, and belongs to the field of the implant materials for mucocutaneous operations. The implant material provided by the invention is characterized in that a non-anti-coagulation blood sample is employed, and centrifuged by a centrifuge with centrifugal force ranging from 130 to 460g, the upper total yellow liquid is put into standing at the room temperature for 30-40 minutes, and then the total yellow liquid is pressed into a mask for clinical application by a metal pressing plate. The implant material provided by the invention is high in yield, and can be naturally formed into gel without adding thrombin and calcium ions; as a result, the use risk of biochemical preparations is avoided; the operation of the implant material is simple; the PRF gel product is thick in fibrous protein filaments, good in elasticity and tenacity, strong in tension, high in hardness, slow in degradation, and not easy to break by tearing; almost no damage is caused in the preparation process of hemameba, blood platelet and shed particles of the two; and after being implanted in vivo, the implant material slowly releases various growth factors and anti-inflammatory cytokines to promote wound healing.
Owner:PEKING UNIV SCHOOL OF STOMATOLOGY
Three dimensional healing kit
ActiveUS10213489B2Easy to separateGood effectDental implantsHeavy metal active ingredientsPlateletBiomedical engineering
Invention; is about the kit that enables three-dimensional healing with the platelet-rich fibrin framework support used in hard and soft tissue healing.
Owner:AKMAN SERHAN +1
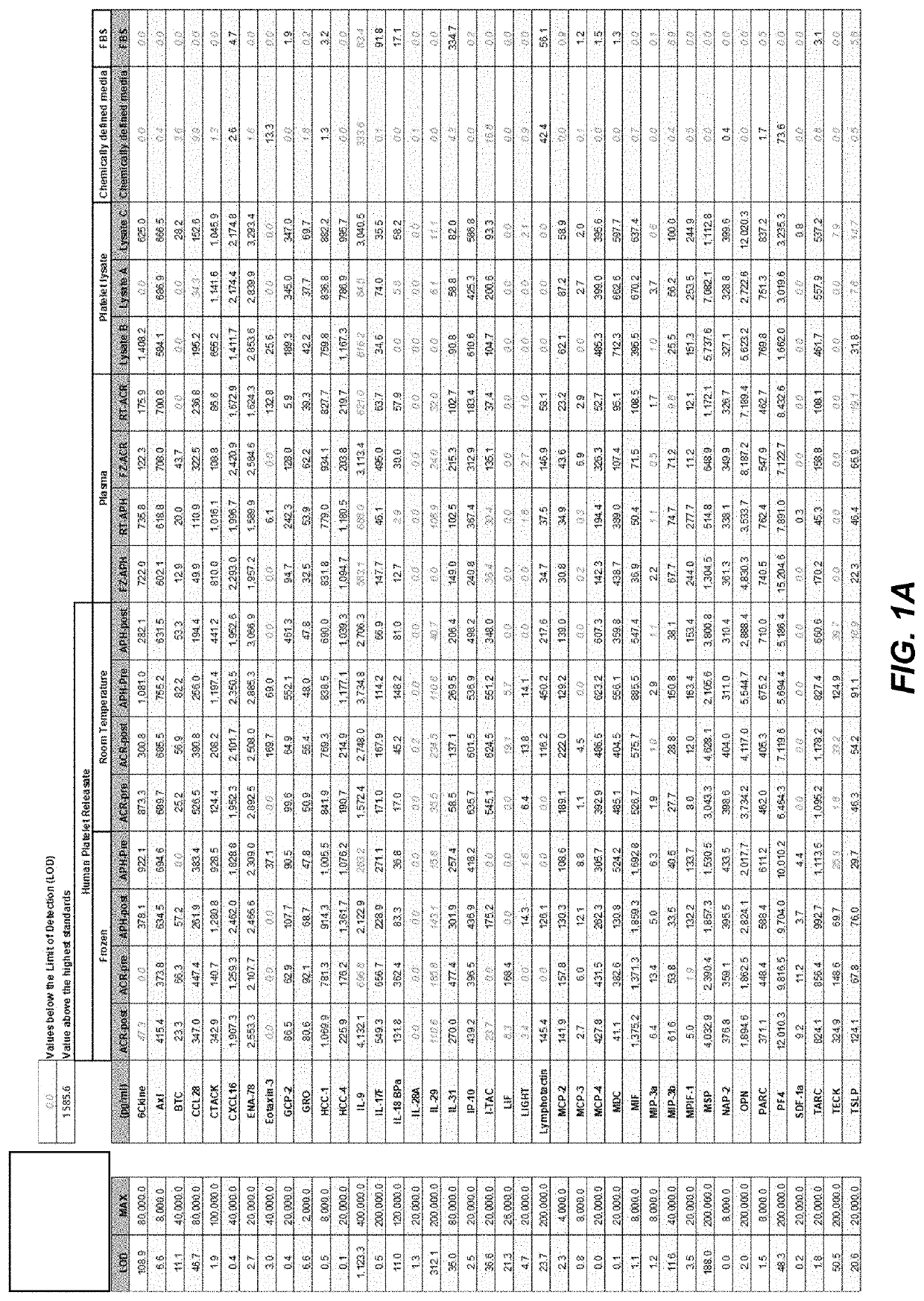
Methods and compositions related to platelet releasate and platelet-rich fibrin
PendingUS20210361718A1Prevent gelationReduce needPeptide/protein ingredientsCulture processHuman plateletAnticoagulant Agent
The present disclosure provides compositions and methods comprising human platelet releasate (hPR), a xeno-free media supplement. The disclosure also relates to a cGMP process for the rapid, efficient and large-scale manufacturing of hPR that may be performed in less than 4 hours. The platelet releasate of the current disclosure prevents gelation of growth media alleviating the need for heparin or other anticoagulants. Mesenchymal stem cells expanded in the presence of platelet releasate have demonstrated superior expansion rates and potency compared to commercial supplements including platelet lysates. The releasate has therapeutic and medical applications. The disclosure also relates to compositions and methods related to platelet-rich fibrin.
Owner:BIOBRIDGE GLOBAL
Compositions and methods for platelet enriched fibrin constructs
ActiveUS10849931B2Peptide/protein ingredientsMammal material medical ingredientsWhite blood cellEngineering
Compositions and methods are provided for tissue constructs that promote wound healing. The composition comprises a dimensionally stable fibrin construct for local administration to a wound site or region. In one embodiment, the fibrin construct is a wound healing composition, including components that promote wound healing, such as platelets, growth factors, white blood cells and fibrin clots. In another embodiment, the tissue treatment composition includes (i) aggregated fibrin, (ii) blood cells, and (iii) optionally, growth factors and / or other proteins.
Owner:DEPUY SYNTHES PROD INC
Compositions and methods for platelet enriched fibrin constructs
PendingUS20210060066A1Peptide/protein ingredientsMammal material medical ingredientsWhite blood cellPlatelet
Compositions and methods are provided for tissue constructs that promote wound healing. The composition comprises a dimensionally stable fibrin construct for local administration to a wound site or region. In one embodiment, the fibrin construct is a wound healing composition, including components that promote wound healing, such as platelets, growth factors, white blood cells and fibrin clots. In another embodiment, the tissue treatment composition includes (i) aggregated fibrin, (ii) blood cells, and (iii) optionally, growth factors and / or other proteins.
Owner:DEPUY SYNTHES PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com