Degradable tissue regeneration barrier membrane and preparing method thereof
A technology for tissue regeneration and barrier membranes, applied in the field of degradable tissue regeneration barrier membranes and its preparation, can solve the problems of biodegradable properties that are easy to cause inflammation, aseptic inflammatory reactions of patients, and long time for barrier membrane degradation, etc., to achieve as soon as possible The effect of tissue regeneration speed, inhibition of inflammation, and acceleration of degradation speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
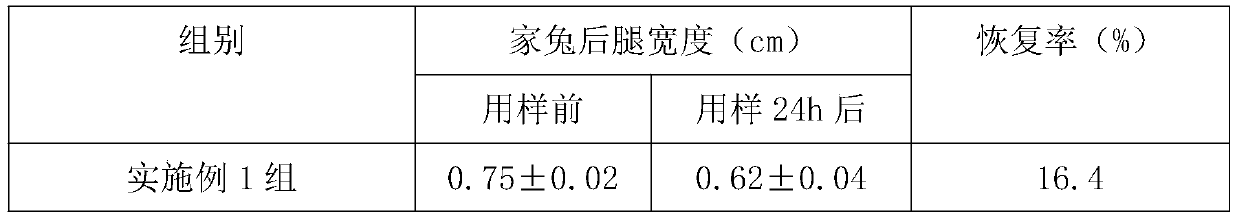
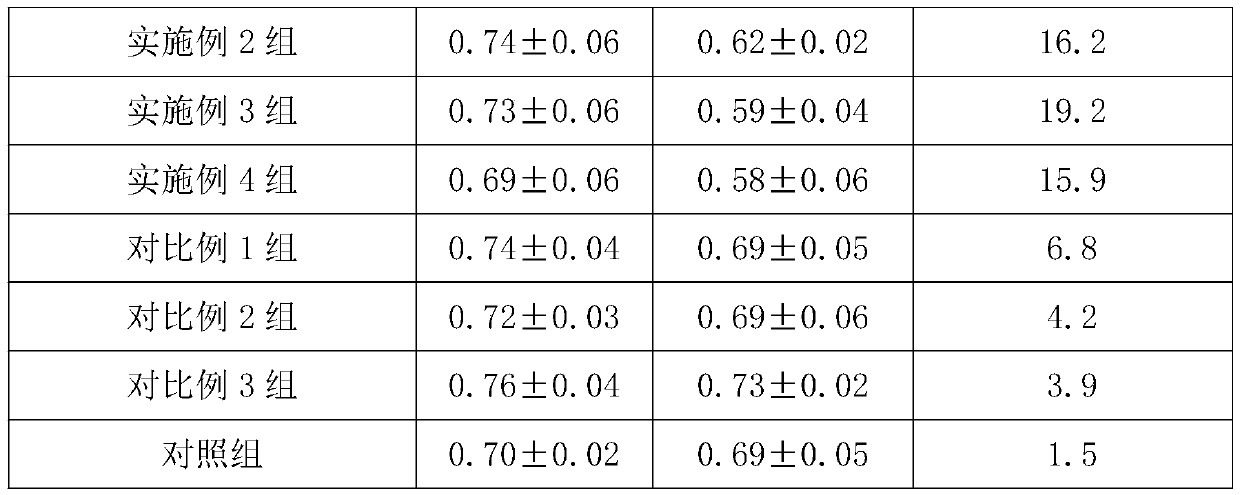
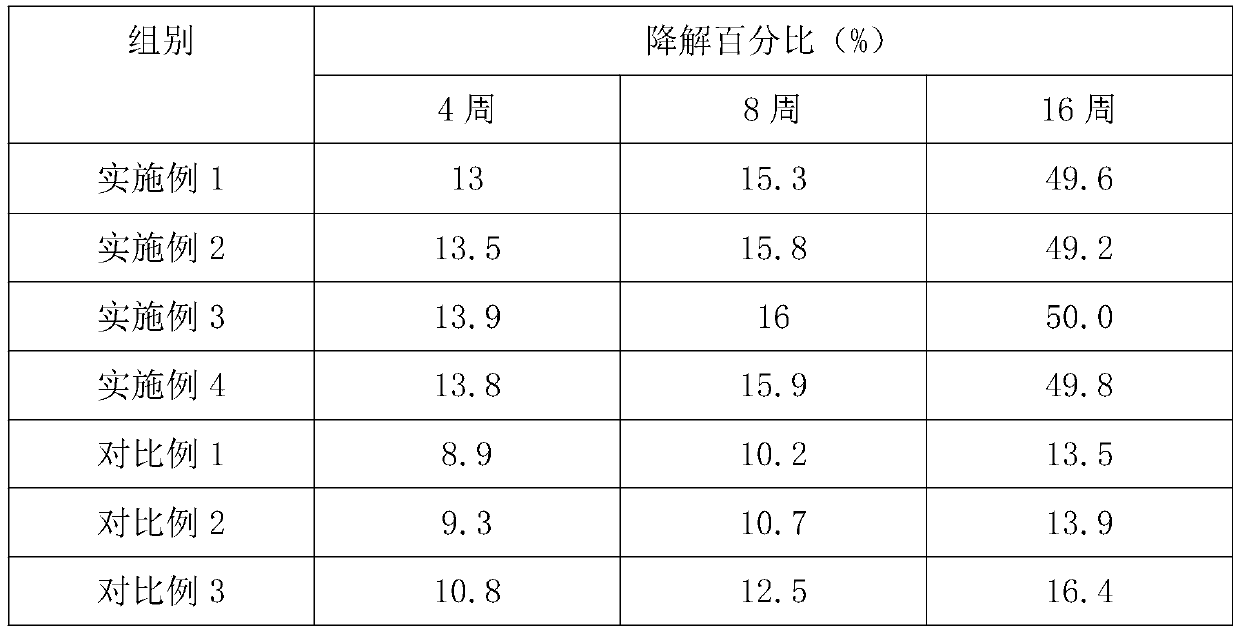
[0034] Embodiment 1, a kind of degradable tissue regeneration barrier film
[0035] The degradable tissue regeneration barrier film is made of the following raw materials and their mass percentages:
[0036] Calcium alginate solution 35%, chitosan solution 30% and platelet-rich fibrin solution 35%.
[0037] The preparation raw material of described calcium alginate solution comprises calcium alginate, distilled water, NaHCO 3 , glacial acetic acid; the preparation raw material of described chitosan solution comprises chitosan, distilled water, glacial acetic acid, lysozyme; The preparation raw material of described platelet-rich fibrin solution comprises platelet-rich fibrin, sodium chloride, distilled water.
[0038] The calcium alginate, distilled water, NaHCO 3 , the mass ratio of glacial acetic acid is 1:15:5:3; the mass ratio of described chitosan, distilled water, glacial acetic acid, lysozyme is 10:500:20:1; described platelet-rich fibrin, sodium chloride , The mass ...
Embodiment 2
[0051] Embodiment 2, a kind of degradable tissue regeneration barrier film
[0052] The degradable tissue regeneration barrier film is made of the following raw materials and their mass percentages:
[0053] Calcium alginate solution 55%, chitosan solution 40% and platelet-rich fibrin solution 5%.
[0054] The preparation raw material of described calcium alginate solution comprises calcium alginate, distilled water, NaHCO 3 , glacial acetic acid; the preparation raw material of described chitosan solution comprises chitosan, distilled water, glacial acetic acid, lysozyme; The preparation raw material of described platelet-rich fibrin solution comprises platelet-rich fibrin, sodium chloride, distilled water.
[0055] The calcium alginate, distilled water, NaHCO 3 , the mass ratio of glacial acetic acid is 1:25:10:10; the mass ratio of described chitosan, distilled water, glacial acetic acid, lysozyme is 20:1500:30:1; described platelet-rich fibrin, sodium chloride , The mas...
Embodiment 3
[0068] Embodiment 3, a kind of degradable tissue regeneration barrier film
[0069] The degradable tissue regeneration barrier film is made of the following raw materials and their mass percentages:
[0070] Calcium alginate solution 40%, chitosan solution 35% and platelet-rich fibrin solution 25%.
[0071] The preparation raw material of described calcium alginate solution comprises calcium alginate, distilled water, NaHCO 3 , glacial acetic acid; the preparation raw material of described chitosan solution comprises chitosan, distilled water, glacial acetic acid, lysozyme; The preparation raw material of described platelet-rich fibrin solution comprises platelet-rich fibrin, sodium chloride, distilled water.
[0072] The calcium alginate, distilled water, NaHCO 3 , the mass ratio of glacial acetic acid is 1:20:7:5; the mass ratio of described chitosan, distilled water, glacial acetic acid, lysozyme is 15:1000:25:1; described platelet-rich fibrin, sodium chloride , The mass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com