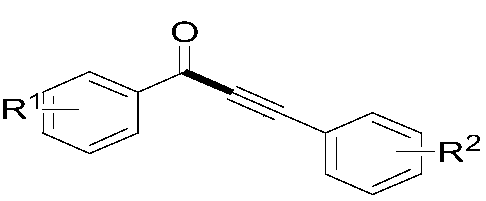
Preparation method of 1, 3-diarylpropargyl ketone
A technology for diarylpropynone and alkynoic acid, which is applied in the field of preparation of 1,3-diarylpropynone and achieves the effects of good compatibility, mild reaction conditions and simple catalytic system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~14
[0033] The α-keto acid (1.1mmol, R 1 =H), aryl alkynoic acid (1mmol, R 2=H), silver catalyst (5mol%) and oxidizing agent (2mmol) were dispersed in the solvent, and then stirred at 50° C. for 3 hours under air atmosphere. After the reaction, with Et 2 O (5 mL) was extracted three times, and the combined organic phases were concentrated under reduced pressure. The obtained crude product was subjected to column chromatography (300-400 mesh silica gel, petroleum ether and ethyl acetate as eluents) to obtain the target product. The silver catalyst, oxidant and solvent used and the reaction results are shown in Table 1.
[0034] The reaction condition and reaction result of table 1 embodiment 1~10
[0035]
[0036]
[0037] a The total volume of solvent was kept at 2 mL.
Embodiment 14~29
[0039] α-keto acid (1.1mmol), aryl alkynoic acid (1mmol, R 2 =H), AgOAc (5mol%) and (NH 4 ) 2 S 2 o 8 (2mmol) dispersed in DMSO (1mL) and H 2 O (1 mL) in a mixed solvent, and then stirred at 50° C. for 3 hours under air atmosphere. After the reaction, with Et 2 O (5 mL) was extracted three times, and the combined organic phases were concentrated under reduced pressure. The obtained crude product was subjected to column chromatography (300-400 mesh silica gel, petroleum ether and ethyl acetate as eluents) to obtain the target product. The substrates used and the reaction results are listed in Table 2.
[0040] The reaction substrate and result used in table 2 embodiment 14~29
[0041]
Embodiment 30~41
[0043] The α-keto acid (1.1mmol, R 1 =H), aryl alkynoic acid (1mmol, R 2 =H), AgOAc (5mol%) and (NH 4 ) 2 S 2 o 8 (2mmol) dispersed in DMSO (1mL) and H 2 O (1 mL) in a mixed solvent, and then stirred at 50° C. for 3 hours under air atmosphere. After the reaction, with Et 2 O (5 mL) was extracted three times, and the combined organic phases were concentrated under reduced pressure. The obtained crude product was subjected to column chromatography (300-400 mesh silica gel, petroleum ether and ethyl acetate as eluents) to obtain the target product. The substrates used and the reaction results are listed in Table 3.
[0044] The reaction substrate and result used in table 3 embodiment 30~41
[0045]
[0046] The characterization data of some products are as follows:
[0047] 1,3-Diphenylprop-2-yn-1-one:White solid. 1 H NMR (400MHz, CDCl 3 )δ8.08(d, J=7.2Hz, 2H), 7.54(d, J=6.8Hz, 2H), 7.50–7.44(m, 1H), 7.38(t, J=7.6Hz, 2H), 7.32( d,J=7.2Hz,1H),7.28–7.26(m,2H). 13 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com