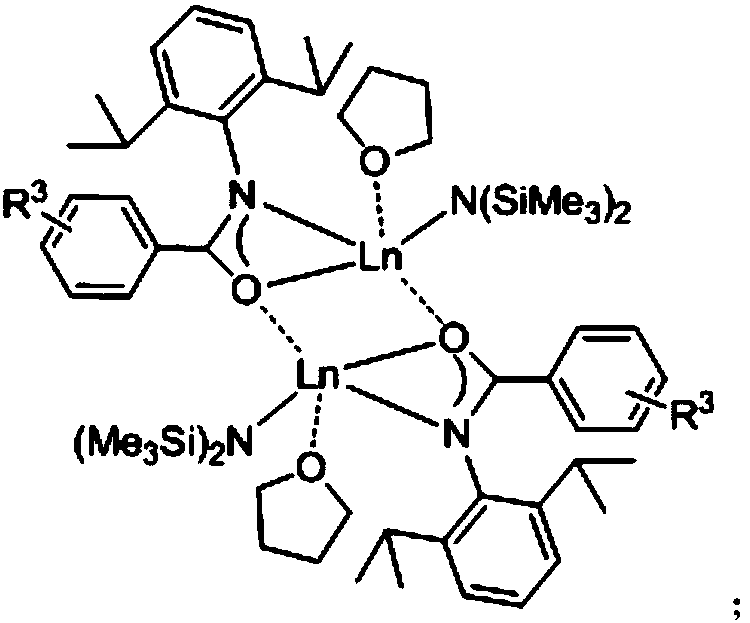
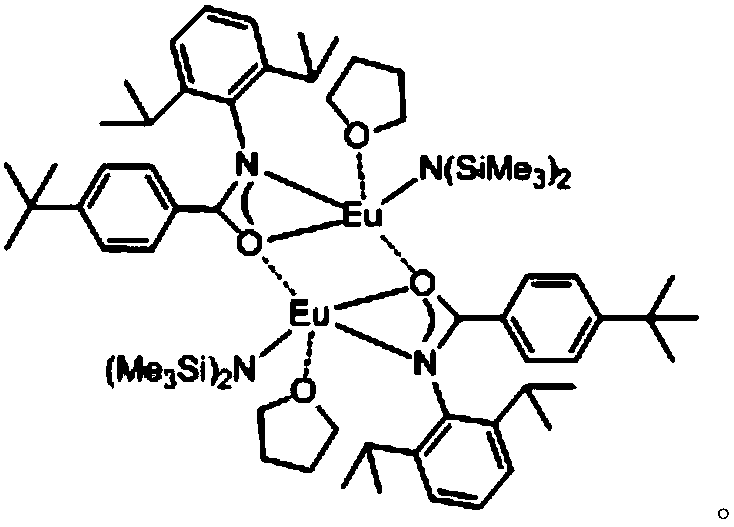
Preparation method of 3, 5-disubstituted thiazolidine-2-thioketone compound
A compound, thiazolidine technology, applied in the field of organic synthesis, can solve the problems of small expansion range of substrates, large amount of catalysts, limited range of substrates, etc., to achieve simple separation and purification of products, high catalytic activity, and wide applicability of reactants Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: 1mol% of {L 1 Eu[N(SiMe 3 ) 2 ]·THF} 2 Catalyzes the reaction of 2-phenyl-1-ethylcycloethyleneimine and carbon disulfide at 45°C:
[0043] Under the airtight conditions of anhydrous, oxygen-free and argon protection, add 0.0144g (1.00×10 -5 mole){L 1 Eu[N(SiMe 3 ) 2]·THF} 2 , then add 147μL (1.00×10 -3 mol) of 2-phenyl-1-ethylcycloethylimine was stirred at room temperature for 5 min under an argon atmosphere, and 300 μL (5.00×10 -3 mol) of carbon disulfide, stirred and reacted in a constant temperature bath at 45°C. After 24 hours, the protective argon gas was removed, and the reaction was quenched by air exposure. Column chromatography was performed using petroleum ether and ethyl acetate as developing solvents, and the solvent was spin-dried to obtain a separation yield of 90%. The structure of the target product is as follows:
[0044]
[0045] NMR data: 1 H NMR (400MHz, CDCl 3 ): δ7.31(s,5H),4.77(s,1H),4.31(s,1H),4.01(s,1H),3.80(s,2H),1.20...
Embodiment 2
[0047] Example 2: 1mol% of {L 1 Eu[N(SiMe 3 ) 2 ]·THF} 2 Catalyze the reaction of 2-phenyl-1-ethylcycloethyleneimine and carbon disulfide at 30°C:
[0048] Under the airtight conditions of anhydrous, oxygen-free and argon protection, add 0.0144g (1.00×10 -5 mole){L 1 Eu[N(SiMe 3 ) 2 ]·THF} 2 , then add 147μL (1.00×10 -3 mol) of 2-phenyl-1-ethylcycloethylimine was stirred at room temperature for 5 min under an argon atmosphere, and 300 μL (5.00×10 -3 mol) of carbon disulfide, stirred and reacted in a constant temperature bath at 30°C. After 24 hours, the protective argon gas was removed, and the reaction was quenched by air exposure. Column chromatography was performed using petroleum ether and ethyl acetate as developing solvents, and the solvent was spin-dried to obtain a separation yield of 72%.
Embodiment 3
[0049] Example 3: 1mol% of {L 1 Eu[N(SiMe 3 ) 2 ]·THF} 2 Catalyzes the reaction of 2-phenyl-1-ethylcycloethyleneimine and carbon disulfide at 40°C:
[0050] Under the airtight conditions of anhydrous, oxygen-free and argon protection, add 0.0144g (1.00×10 -5 mole){L 1 Eu[N(SiMe 3 ) 2 ]·THF} 2 , then add 147μL (1.00×10 -3 mol) of 2-phenyl-1-ethylcycloethylimine was stirred at room temperature for 5 min under an argon atmosphere, and 300 μL (5.00×10 -3 mol) of carbon disulfide, stirred and reacted in a constant temperature bath at 40°C. After 24 hours, the protective argon gas was removed, and the reaction was quenched by air exposure. Column chromatography was performed with petroleum ether and ethyl acetate as developing solvents, and the solvent was spin-dried to obtain a separation yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com