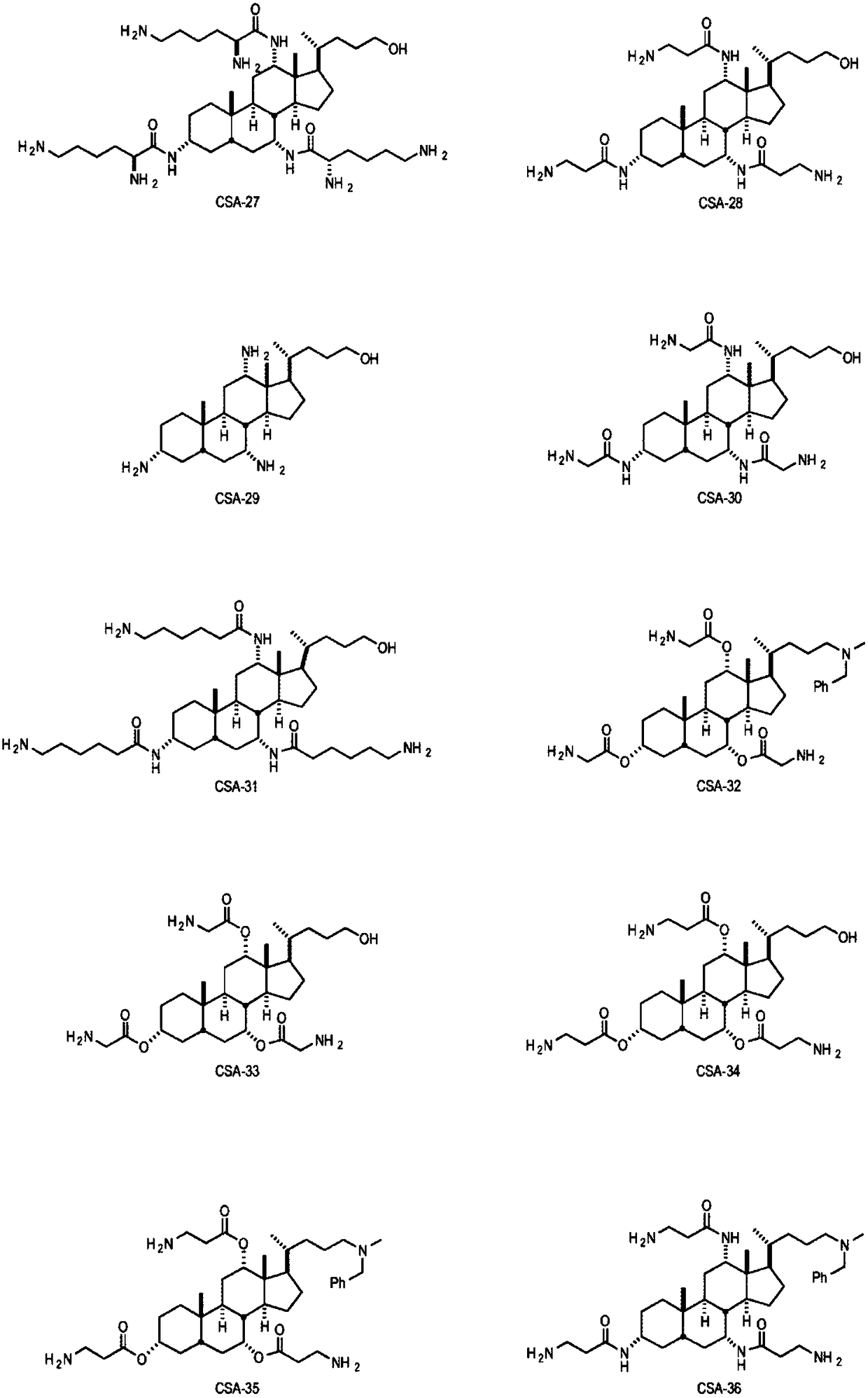
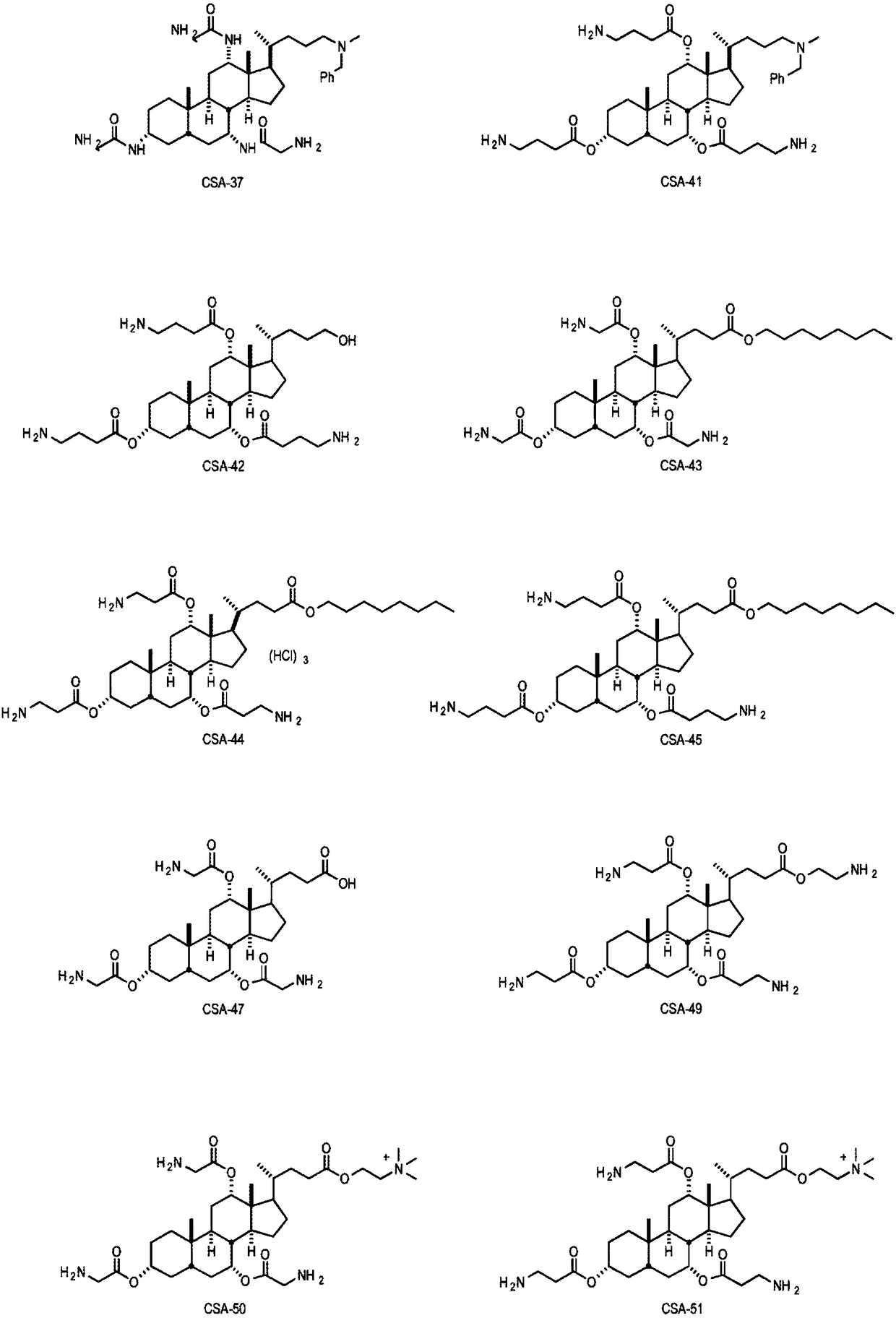
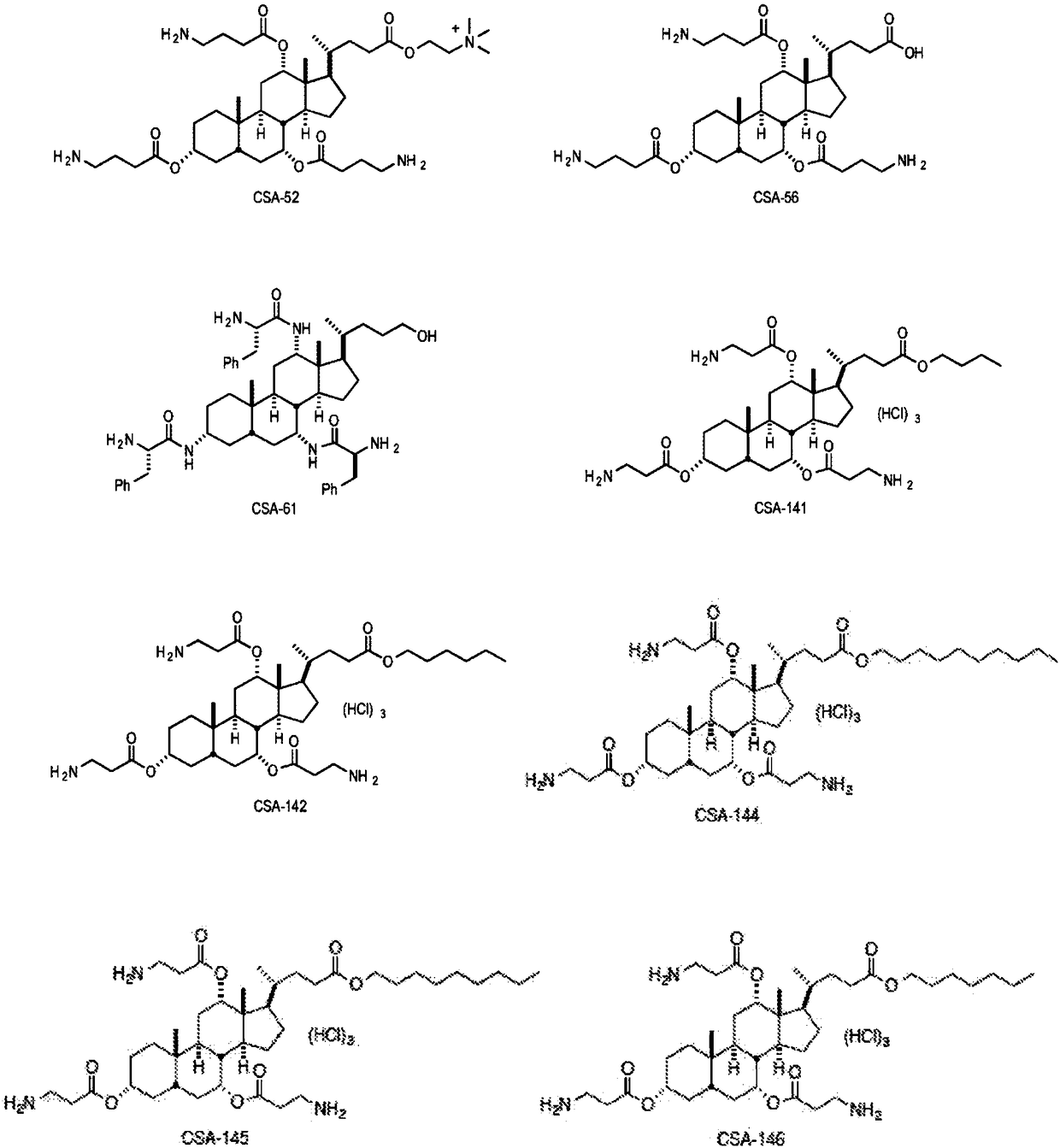
Medical devices integrating cationic steroidal antimicrobial compounds
A medical device, anti-microbial technology, applied in the field of medical devices to achieve the effect of reducing inflammation, reducing contamination of implants, and reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] To determine the role of synthetic seraginines CSA-13, 44 and 90 in inflammation, mesenchymal stem cells (MSCs), targeted mRNA panels from SABiosciences and primary cells from Lonza were used. Cells were purchased from Lonza.com and used fresh for each test, using recommended media and culture conditions. After processing, use Qiagen RNeasy Mini Isolate mRNA and use NanoDrop Purity was quantified by UV at 260 nm and 260 / 280 ratio. Using First Strand from SABiosciences cDNA was prepared and real-time PCR was performed using a kit from the same company for selective analysis of wound healing pathways. The q-PCR results were uploaded to SABiosciences website and Ingenuity.com website for analysis and pathway drawing. On day 1, primary human cells were cultured using 6-well plates with 3 ml of the recommended medium - hMSCBasal Medium + BulletKit (50 ml Growth Supplement, 10 ml L-Glutamine, and 0.5 ml Gentamicin Sulfate Amphotericin-B). MSC cells were plated at a den...
Embodiment 2
[0082] IL-6 is a marker of systemic inflammation. A nonlethal dose of Pseudomonas aeruginosa (P. aeruginosa) was used to infect the respiratory tract of female C57 / BL6 mice as a pneumonia model. One cohort (n=6) also received 80 mg / kg CSA-13; the second cohort (n=6) also received 40 mg / kg CSA-13; the third cohort (n=6) did not receive CSA treatment; the fourth cohort ( n=6) Not infected. Examination of IL-6 levels in kidneys 24 hours post-infection revealed that those infected animals without CSA treatment had IL-6 levels >15-fold that of controls and 5-10-fold higher than those in CSA-treated animals. Thus, treatment with CSA significantly reduced renal IL-6 levels in a pneumonia model.
Embodiment 3
[0084] Silicone-based Foley catheters were coated with an approximately 10 μm thick hydrogel coating. Coatings include CSA-131. Initially, the coating was shown to maintain efficacy for about 6 or 7 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com