A kind of substituted naphthalene ring compound, pharmaceutical composition and application thereof
A compound and composition technology, applied in the field of medicine, to achieve the effect of improving applicability, good pharmacokinetic parameter characteristics, and increasing drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
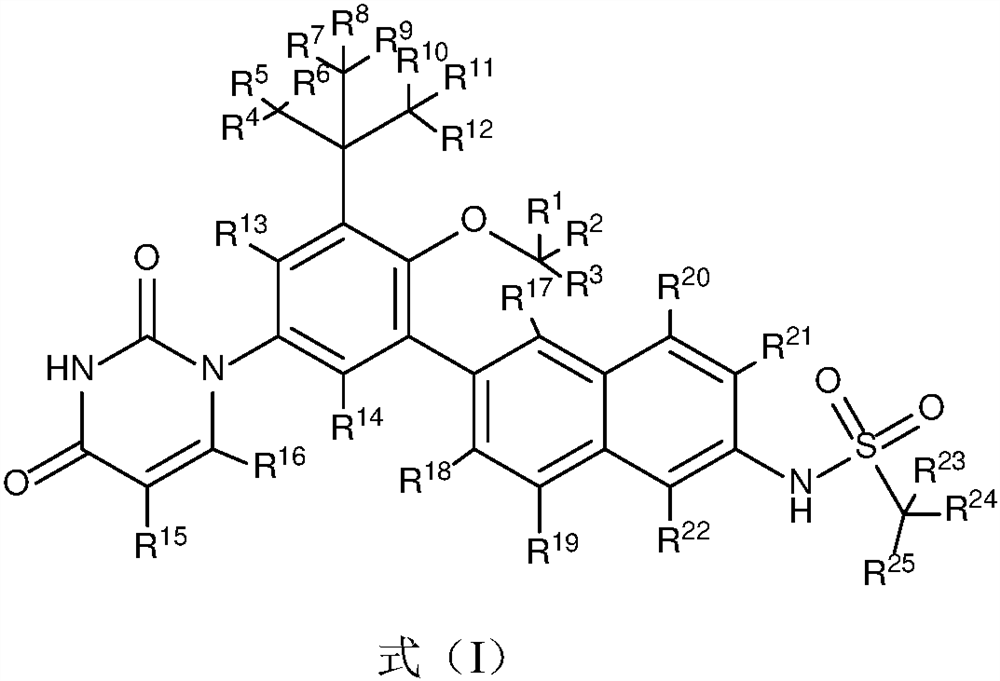
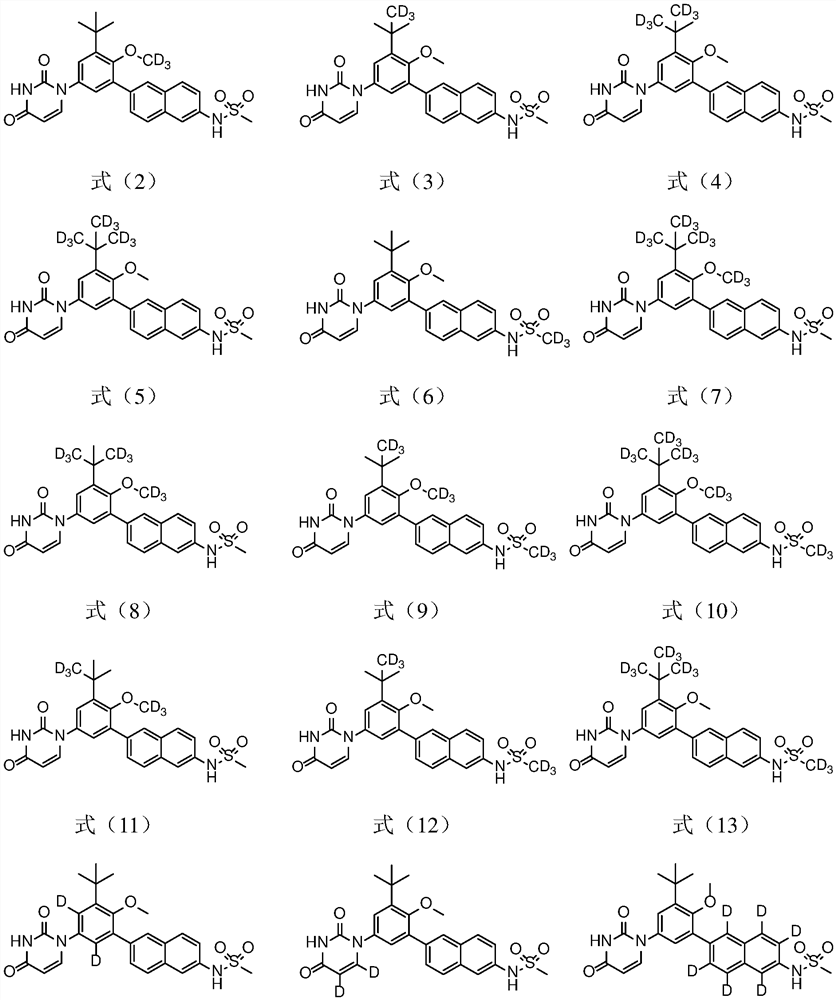
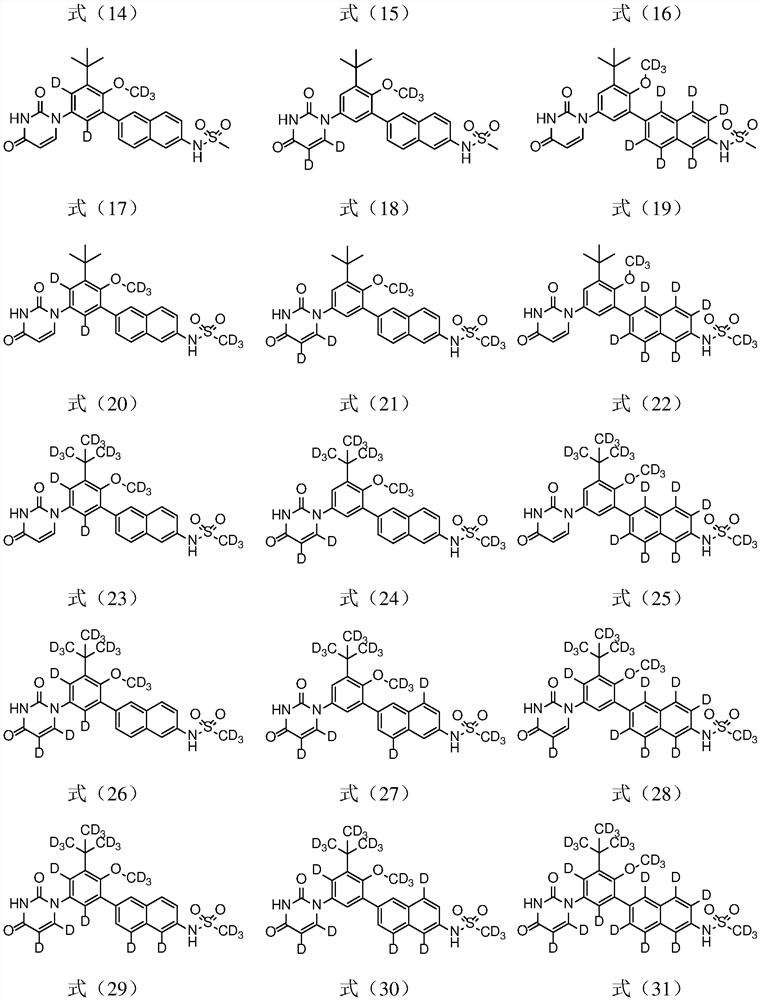
[0055] Example 1 Preparation of N-(6-(3-tert-butyl-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-d3- Methoxyphenyl)naphthalen-2-yl)methanesulfonamide (Compound D-1)
[0056]
[0057] Concrete synthetic steps are as follows:
[0058]
[0059] Step 1. Synthesis of N-(2-cyanophenyl)pyridineamide (Compound 3).
[0060] Add pyridine-2-carboxylic acid (1.23g, 10mmol), 2-cyanoaniline (1.42g, 12mmol), 2-(7-benzotriazole oxide)-N, N, N', N'-tetramethyluronium hexafluorophosphate (HATU, 7.6g, 20mmol) and N,N-diisopropylethylamine (DIPEA, 5.17g, 40mmol), were dissolved in 50mL DMF, stirred at room temperature to react 4- After 6 hours, after the reaction was detected by TLC, ethyl acetate was added for dilution, washed successively with 5% aqueous citric acid solution, water, sodium bicarbonate solution and saturated brine, concentrated, and recrystallized by adding a small amount of isopropanol to obtain 1.23 g of the product. Rate: 55.15%.
[0061] Step 2. Synthesis of 6-hy...
Embodiment 2
[0076] The compounds of the above examples were evaluated for biological activity.
[0077] In order to verify the effect of the compounds described herein on HCV, the inventors used the HCV Replicon System (HCV Replicon System) as an evaluation model. Since it was first reported by Science in 1999, the HCV replicon system has become one of the most important tools for studying HCV RNA replication, pathogenicity and virus persistence. -NCR minimal region, and the HCV replicon system has been successfully used as a model for the evaluation of antiviral drugs. The inventors of the present invention conducted verification according to the methods described in Science, 1999, 285(5424), 110-3, and J. Virol, 2003, 77(5), 3007-19.
[0078] (1) Detect compound anti-HCV 1a and 1b genotype replicon activity
[0079] HCV-1a and HCV-1b stably transfected replicon cells were used to detect the inhibitory activity of compounds on HCV genotype 1a and 1b replicon. In this experiment, Dasab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com