Preparation method of adsorbent and application of adsorbent to heavy metal adsorption
An adsorbent, heavy metal technology, applied in chemical instruments and methods, adsorbed water/sewage treatment, other chemical processes, etc., can solve problems such as poor adsorption capacity, and achieve enhanced adsorption capacity, increased specific surface area, and good hydrothermal stability. Effects of sex and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
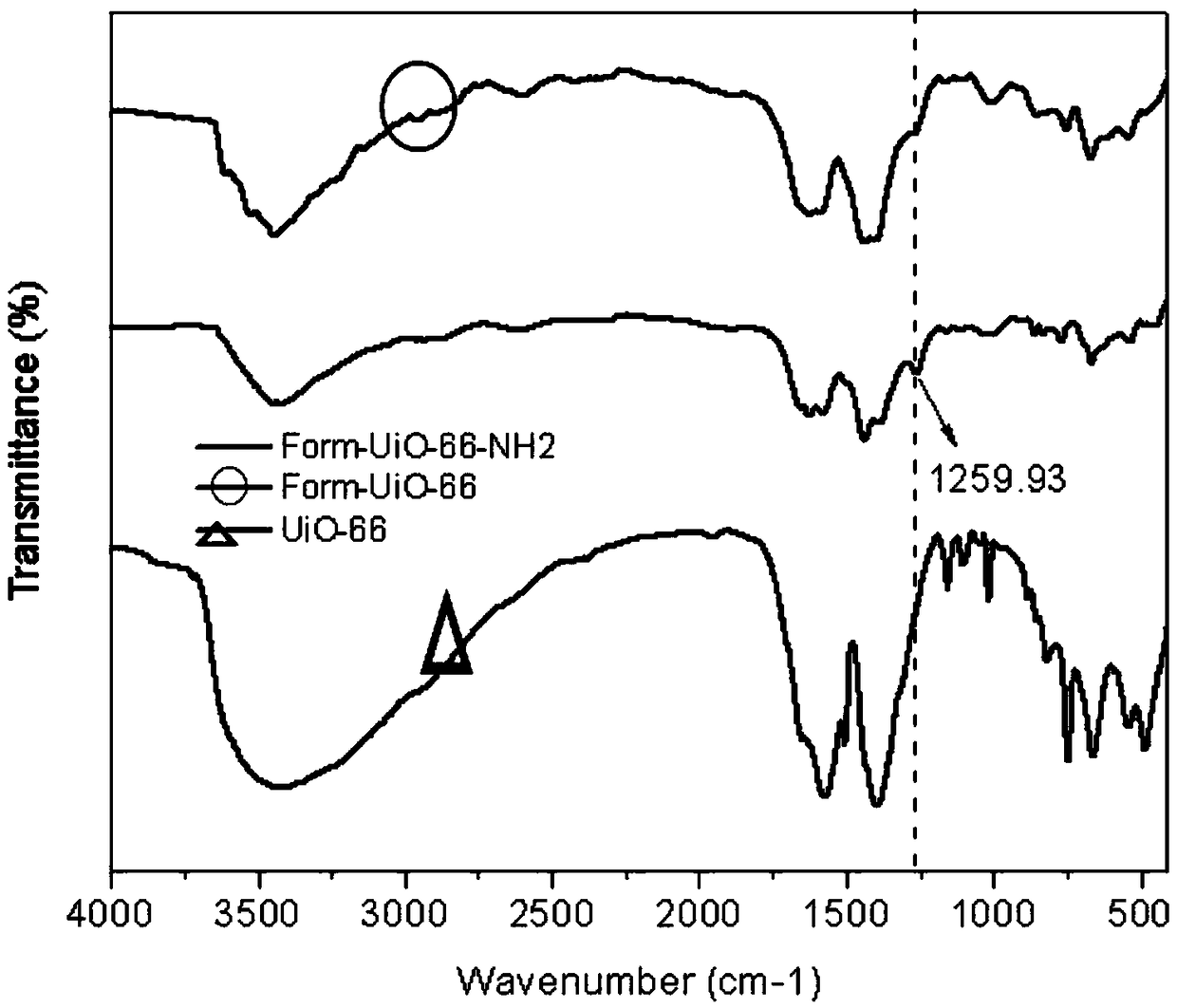
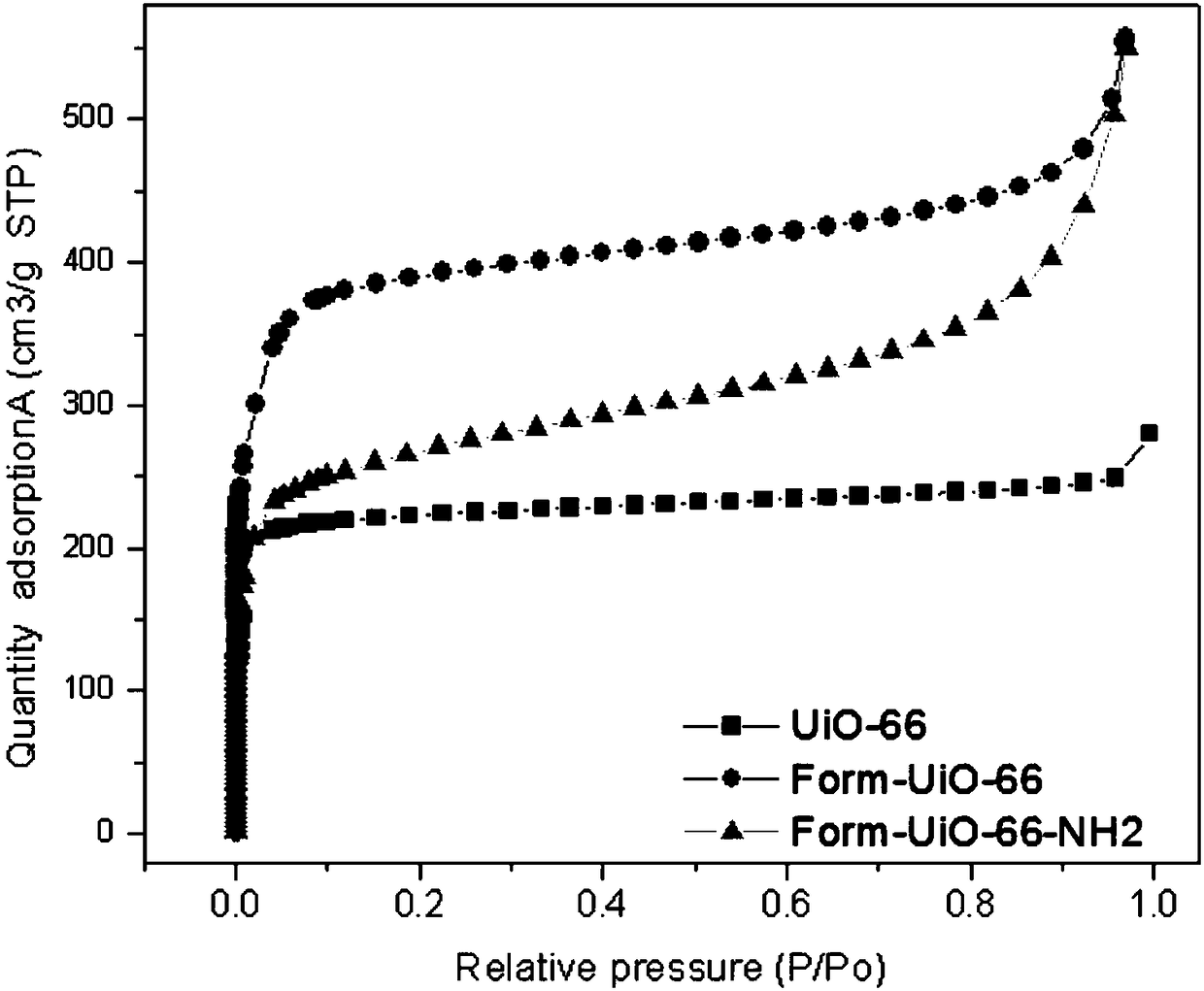
Image
Examples
Embodiment 1
[0035] This embodiment is to prepare Form-UiO-66-NH 2 Specific examples of adsorbent product 1.
[0036] Weigh 0.85g of zirconium chloride and 0.65g of 2-aminoterephthalic acid into a glass beaker, add solvent 1, and heat at 60°C for 1.5h to obtain the first product 1. Among them, solvent 1 is composed of 95ml N,N-dimethylformamide (DMF), 0.5ml ultrapure water and 8.5ml formic acid.
[0037] The first product 1 was transferred to a polyethylene tetrafluoro reactor, placed in a constant temperature oven, and subjected to a hydrothermal synthesis reaction at 110° C. for 72 hours to obtain the second product 1 .
[0038] The second product 1 was cooled, washed with DMF, dried overnight at 65° C., ground for 15 min and sieved to obtain the third product 1 .
[0039] The third product 1 was activated at 190°C for 22 hours in a constant temperature oven to obtain Form-UiO-66-NH 2 Adsorbent product 1.
Embodiment 2
[0041] This embodiment is to prepare Form-UiO-66-NH 2 Specific examples of adsorbent product 2.
[0042] Weigh 0.9g of zirconium chloride and 0.7g of 2-aminoterephthalic acid into a glass beaker, add solvent 2, and heat at 70°C for 2h to obtain the first product 2. Among them, solvent 2 is composed of 105ml N,N-dimethylformamide (DMF), 0.2ml ultrapure water and 9ml formic acid.
[0043] The first product 2 was transferred to a polyethylene tetrafluoro reactor, placed in a constant temperature oven, and subjected to a hydrothermal synthesis reaction at 120° C. for 60 hours to obtain the second product 2 .
[0044] The second product 2 was cooled, washed with DMF, dried overnight at 60° C., ground for 30 min and sieved to obtain the third product 2 .
[0045] The third product 2 was activated at 210°C for 24 hours in a constant temperature oven to obtain Form-UiO-66-NH 2 Adsorbent product 2.
Embodiment 3
[0047] This embodiment is to prepare Form-UiO-66-NH 2 Specific examples of adsorbent product 3.
[0048]Weigh 0.65g of zirconium chloride and 0.66g of 2-aminoterephthalic acid into a glass beaker, add solvent 3, and heat at 65°C for 1 hour to obtain the first product 3. Among them, solvent 3 is composed of 100ml N,N-dimethylformamide (DMF), 0.3ml ultrapure water and 8.6ml formic acid.
[0049] The first product 3 was transferred to a polyethylene tetrafluoro reactor, placed in a constant temperature oven, and subjected to a hydrothermal synthesis reaction at 115° C. for 74 hours to obtain the second product 3 .
[0050] The second product 3 was cooled, washed with DMF, dried overnight at 70° C., ground for 20 min and sieved to obtain the third product 3 .
[0051] The third product 3 was activated at 205°C for 20 hours in a constant temperature oven to obtain Form-UiO-66-NH 2 Adsorbent products3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com