Method for synthesizing allyl sulfides by means of direct functionalization of C-H bonds
An allyl and sulfide technology, applied in chemical instruments and methods, physical/chemical process catalysts, sulfide preparation, etc., to achieve the effects of reducing reaction steps, reducing costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
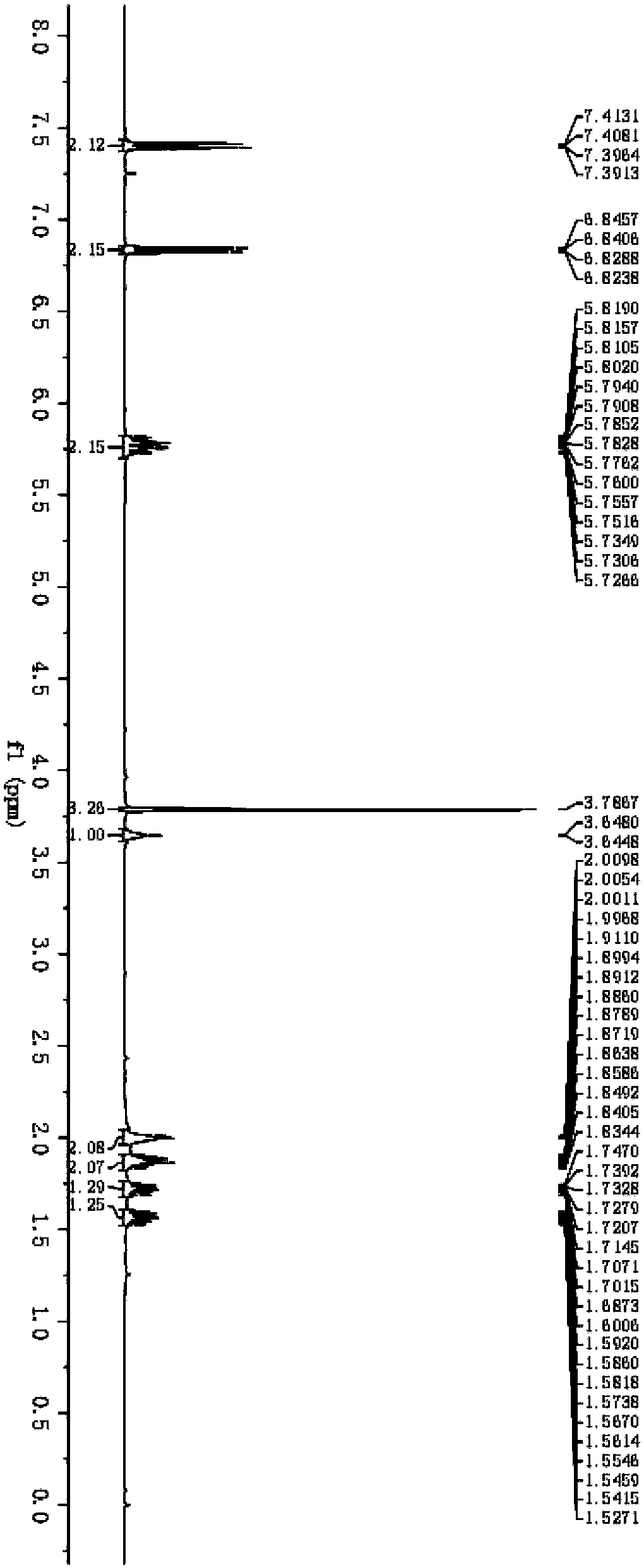
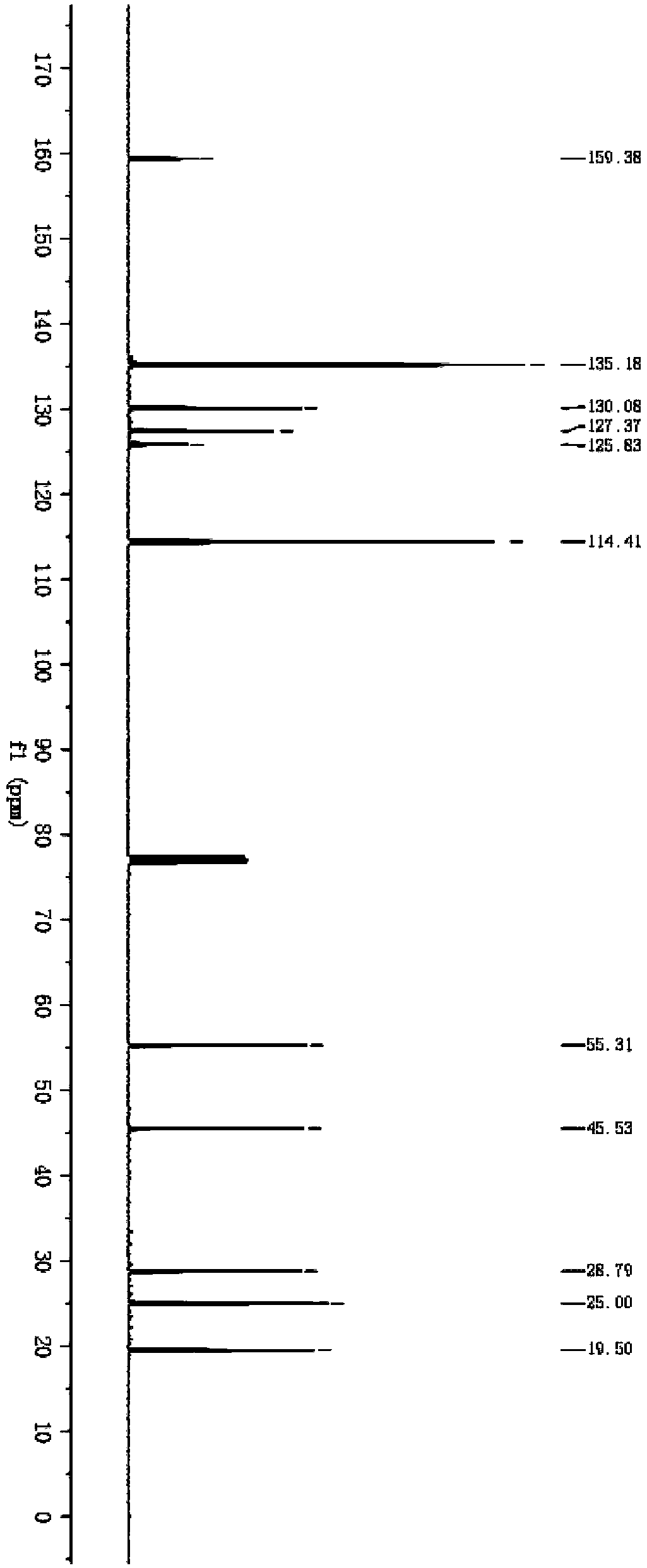
[0042] The synthesis of compound cyclohexyl-2-en-1-yl (4-methoxyphenyl) sulfane, the steps are as follows:
[0043] 1) Take 7mL concentration to be 8.0×10 -5 mol / L CdSe quantum dot aqueous solution, adding 0.1mL concentration is the mixed solution of nitric acid of 2mol / L, this mixed solution is centrifuged on the centrifuge, removes the upper layer aqueous solution, obtains solid substance; The purpose of this step is (1) to remove Ligands on the surface of quantum dots; (2) remove the solvent water by centrifugal precipitation to obtain solid matter, which is beneficial to use other solvents for research.
[0044] 2) Add the solid substance in step 1) to 4 mL of acetonitrile, and ultrasonicate for 2 minutes to obtain a clear mixed solution of acetonitrile;
[0045] 3) Add the mixed solution in step 2) to a 10mL test tube, and add 0.1mmol 4-methoxythiophenol and 5mmol cyclohexene to the acetonitrile mixed solution to obtain a reaction solution;
[0046] 4) Under the protect...
Embodiment 2
[0051] The effect of the reaction under the air condition and under the protection condition of argon was compared. The steps of the method are the same as in Example 1, except that the conditions in step 4) are changed, that is, under air conditions, the reaction solution in step 3) is irradiated with LED blue light for 20 h. The results are shown in Table 1:
[0052] Table 1 reacts effect comparison under air condition and argon protection condition
[0053] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com