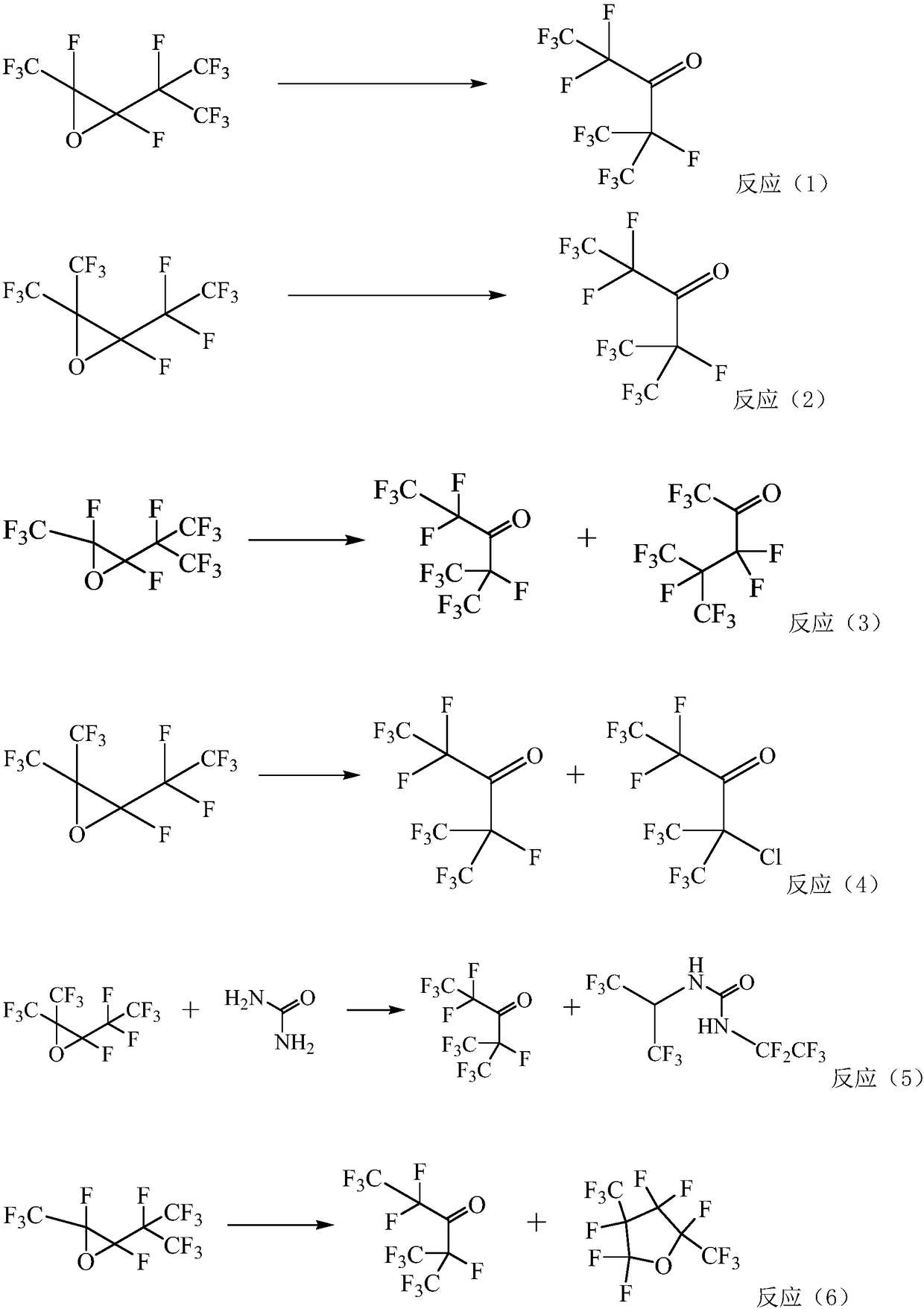
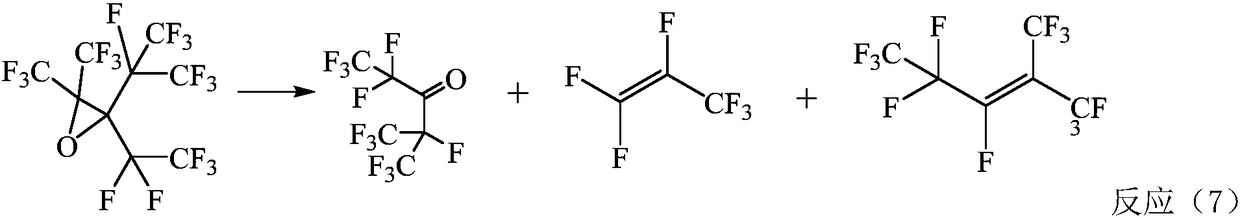
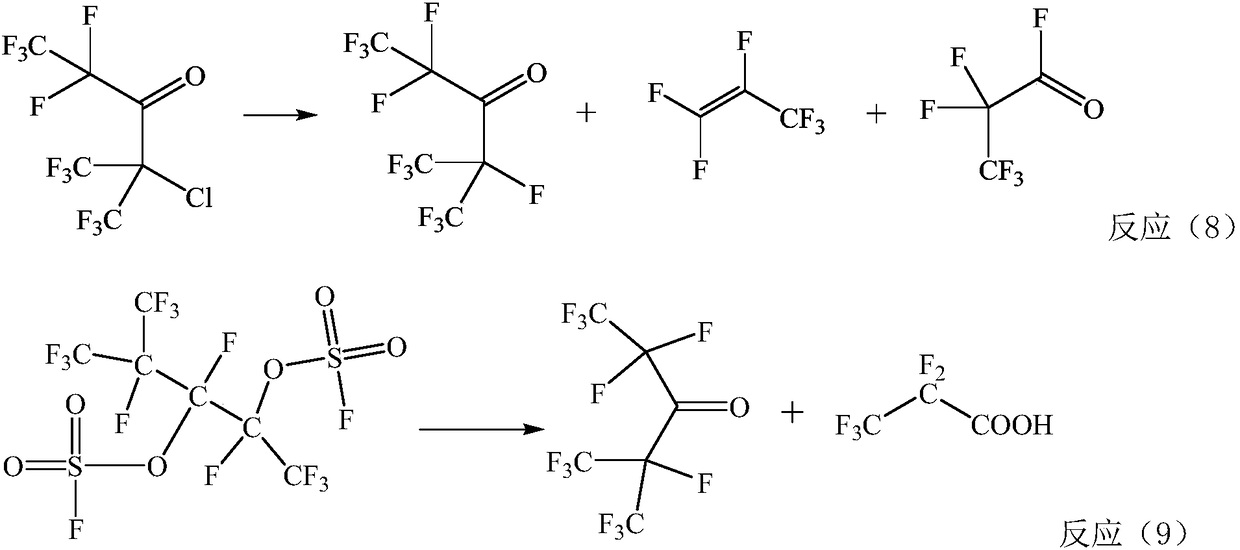
Method for preparing pentafluoride ethyl perfluoro isopropyl ketone through gas phases
A technology of perfluoroisopropyl ketone and pentafluoroethyl, which is applied in the field of gas phase preparation of pentafluoroethyl perfluoroisopropyl ketone, can solve the problems of difficulty in obtaining raw materials, many types of fragments, low yield and the like, and achieves high price low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Fill 10 milliliters of the addition catalyst 10%CsF / 90%CrF that the above-mentioned method prepares in the tubular reactor made of Incon alloy of inner diameter 1 / 2 inch, long 30cm 3 . The reaction conditions are as follows: the reaction temperature is raised to 160°C, the molar ratio of hexafluoropropylene oxide to hexafluoropropylene is 1:1, the contact time is 0.25s, and the reaction pressure is 0.1MPa. The reaction product was collected by a sampling bag made of polytetrafluoroethylene. After 10 hours of reaction, a sample was taken from the sampling bag for GC analysis. The reaction result was: the conversion rate of hexafluoropropylene oxide was 100%, and the conversion rate of pentafluoroethyl perfluoro The selectivity of isopropyl ketone is 99.6%.
[0048] After rectification, high-purity pentafluoroethyl perfluoroisopropyl ketone was isolated, and the boiling point of pentafluoroethyl perfluoroisopropyl ketone was 49.2°C (pressure 760mmHg).
Embodiment 2
[0050] The isomerization catalyst 10%CsF / 90%AlF that 10 milliliters of above-mentioned methods are prepared is packed in the tubular reactor that inner diameter 1 / 2 inch, length 30cm make of Incon alloy 3 . The reaction conditions are as follows: the reaction temperature is raised to 140° C., the contact time of hexafluoropropylene oxide is 0.25 s, and the reaction pressure is 0.1 MPa. The reaction product was collected by a sampling bag made of polytetrafluoroethylene. After 10 hours of reaction, a sample was taken from the sampling bag for GC analysis. The reaction results were as follows: the conversion rate of hexafluoropropylene oxide was 100%, and the conversion rate of pentafluoropropionyl fluoride was 100%. The selectivity is 99.8%.
[0051] After rectification, high-purity pentafluoropropionyl fluoride was isolated, and the boiling point of pentafluoropropionyl fluoride was -26.5°C (pressure 760mmHg).
Embodiment 3
[0053] Fill 10 milliliters of the addition catalyst 10%CsF / 90%CrF that the above-mentioned method prepares in the tubular reactor made of Incon alloy of inner diameter 1 / 2 inch, long 30cm 3 . The reaction conditions are as follows: the reaction temperature is raised to 160°C, the molar ratio of carbonyl fluoride to hexafluoropropylene is 1:1, the contact time is 0.25s, and the reaction pressure is 0.1MPa. The reaction product was collected by a sampling bag made of polytetrafluoroethylene. After 10 hours of reaction, a sample was taken from the sampling bag for GC analysis. The reaction result was: the conversion rate of hexafluoropropylene was 97.4%, and the selection of heptafluoroisobutyryl fluoride was 97.4%. The sex is 98.8%.
[0054] After rectification, high-purity heptafluoroisobutyryl fluoride is separated and obtained, and the boiling point of heptafluoroisobutyryl fluoride is -2~4°C (pressure is 760mmHg).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com