Method for inspecting microbial limit of nifuratel and nysfungin vaginal soft capsules
A nifuratel nystatin, microbial limit technology, applied in the directions of biochemical equipment and methods, microbial determination/inspection, etc., to achieve the effects of simple operation process, elimination of nifuratel, and reduction of bacteriostatic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
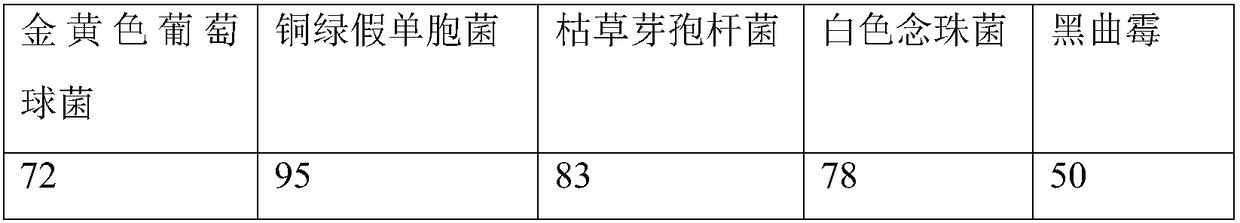
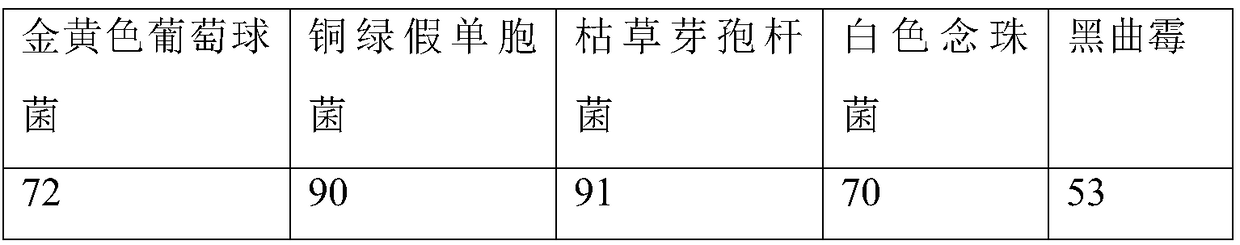
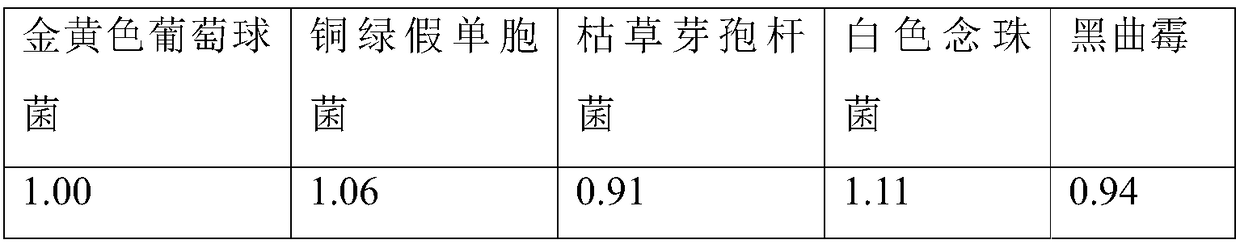
[0041] (1) Bacterial liquid preparation
[0042] ① Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis
[0043] Take fresh cultures of Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis that have been cultured at 30-35°C for 18-24 hours, and use 0.9% sterile sodium chloride solution to make 50-100 cfu per 1ml bacterial suspension.
[0044] ②Candida albicans
[0045] Take the fresh culture of Candida albicans cultured at 20-25°C for 2-3 days, and use 0.9% sterile sodium chloride solution to make a bacterial suspension containing 50-100 cfu per 1 ml.
[0046] ③Aspergillus niger
[0047] Take the fresh culture of Aspergillus niger cultured at 20-25°C for 5-7 days, add 3-5ml of 0.9% sterile sodium chloride solution containing 0.05% (ml / ml) polysorbate 80, elute the spores, and suck out Put the spore suspension into a sterile test tube, and use 0.9% sterile sodium chloride solution to make a spore suspension containing 50-100 cfu of spores per 1 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com