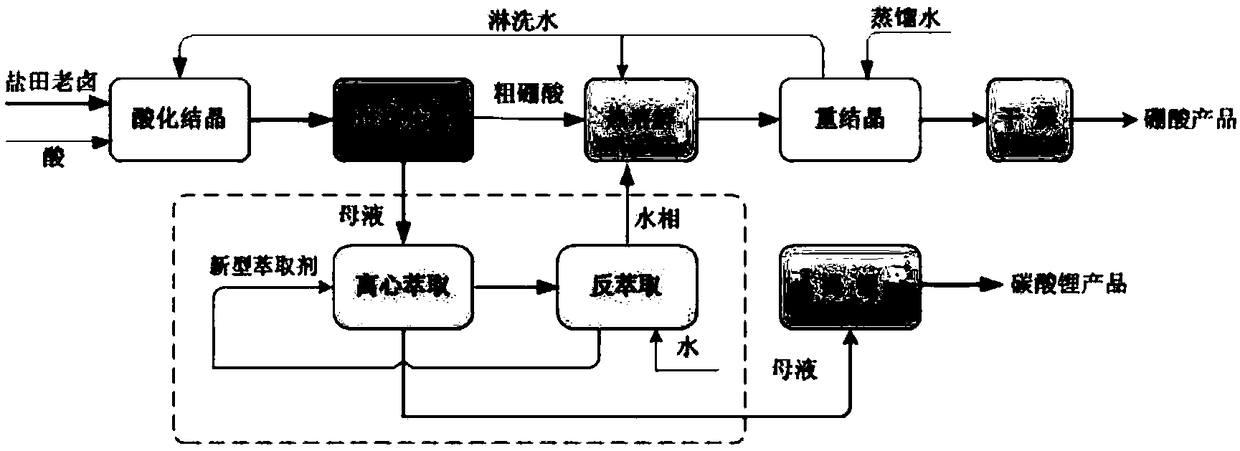
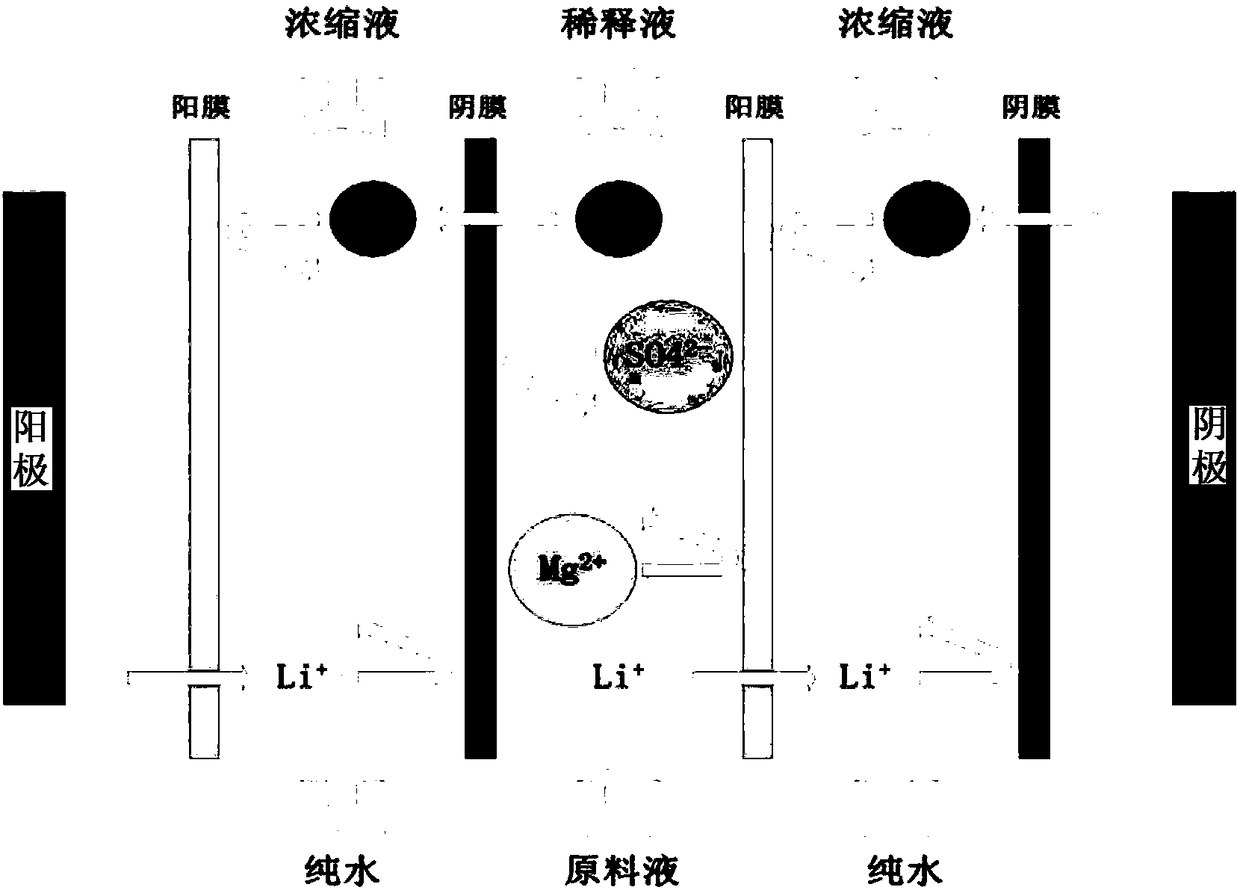
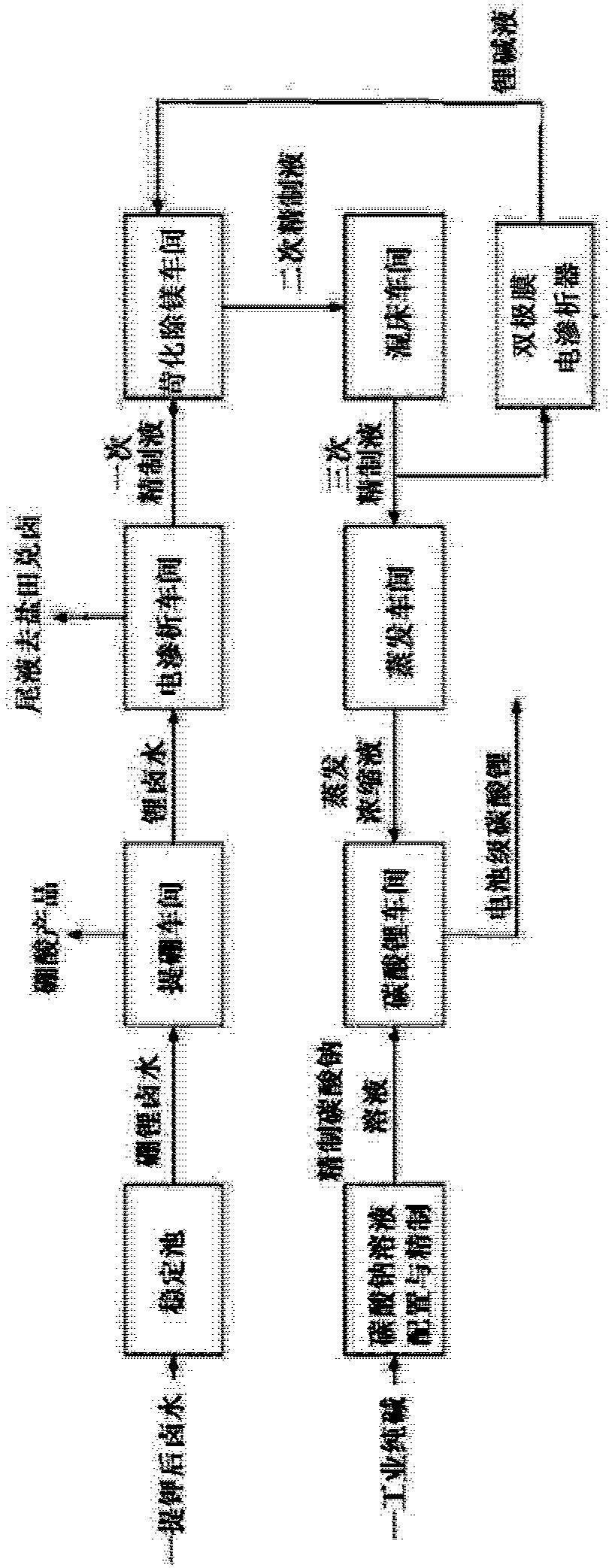
Method for directly preparing lithium carbonate from salt lake brine with high magnesium-to-lithium ratio
A technology of salt lake brine and high magnesium-to-lithium ratio, applied in lithium carbonate;/acid carbonate, chemical instruments and methods, lithium batteries, etc., can solve the problem that there is no universal standard for battery-grade lithium carbonate, and entrainment loss cannot be solved , loss of liquid lithium brine and other problems, to achieve good effect and cost, low separation effect of magnesium and lithium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Take the salt lake intercrystalline brine in Uyuni, Bolivia as an example:
[0127] (1) Form boron-lithium brine
[0128] The intercrystalline brine of the salt lake in Uyuri Salt Lake in Bolivia is extracted for evaporation and concentration in the salt field. During the evaporation process, the tail liquid (mainly magnesium chloride solution) after lithium extraction in the workshop is used for magnesium supplementation in the salt field. After the potassium extraction is formed, the brine is introduced into the stabilization pool for evaporation and stabilization to form a boron-lithium brine with stable components. Focus on monitoring the concentration of magnesium ions and sulfate radicals. In order to save investment, the stabilization pool is also used as a brine storage pool. According to the production plan and ambient temperature and sunshine conditions, the stable components of the brine after potassium extraction are stored by means of adjusting evaporation...
Embodiment 2
[0155] Take the intergranular brine of Rincon Salt Lake in Argentina as an example:
[0156] (1) Collect the intercrystalline brine from Lincoln Salt Lake in Argentina, concentrate it by evaporation, and control it before a large amount of bischofite is precipitated to obtain the boron-lithium brine component. This brine can also be further concentrated to a lithium ion concentration of 6.42g / L and a boron content of 4.65g / L. However, the concentration process will cause the loss of a large amount of lithium precipitated from bischofite and the precipitation of a large amount of lithium sulfate, and the overall loss of lithium ions exceeds 30%.
[0157] Lincoln salt lake (Rincon) intergranular brine composition:
[0158] ingredients
Li +
K +
Na +
Mg 2+
Ca 2+
B 3+
SO 4 2-
Cl -
proportion
g / L
0.40
7.51
115.95
3.42
0.49
0.33
12.52
188.43
1.204
[0159] The brine composition of Lincol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com