Method for obtaining enzyme mutant with high expression, high activity and high stability
A high-stability, mutant technology, applied in the fields of genetic engineering and enzyme engineering, can solve problems such as difficult to obtain thermal stability, and achieve the effect of fast speed, simple screening method and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Construction of a functional polypeptide library
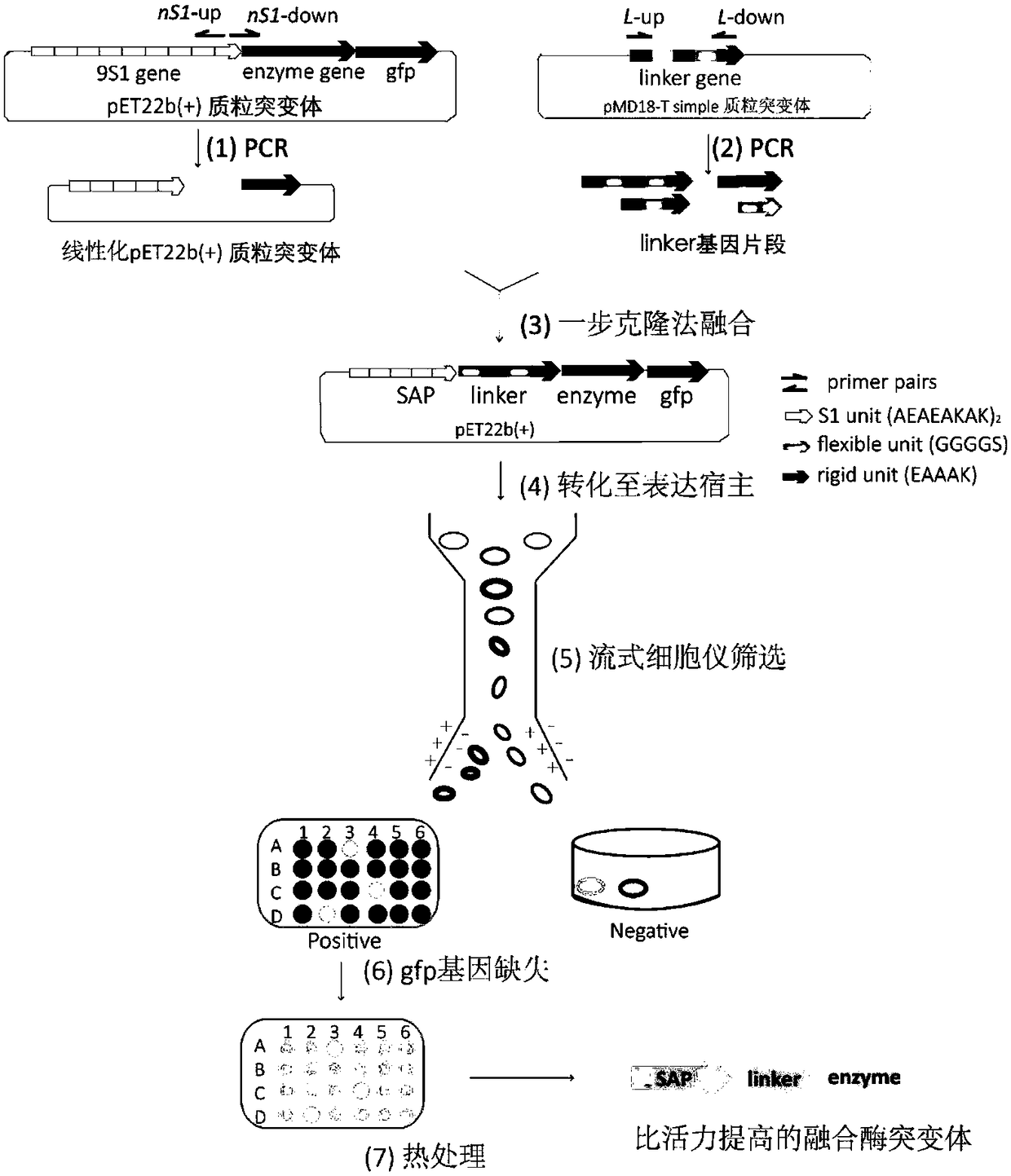
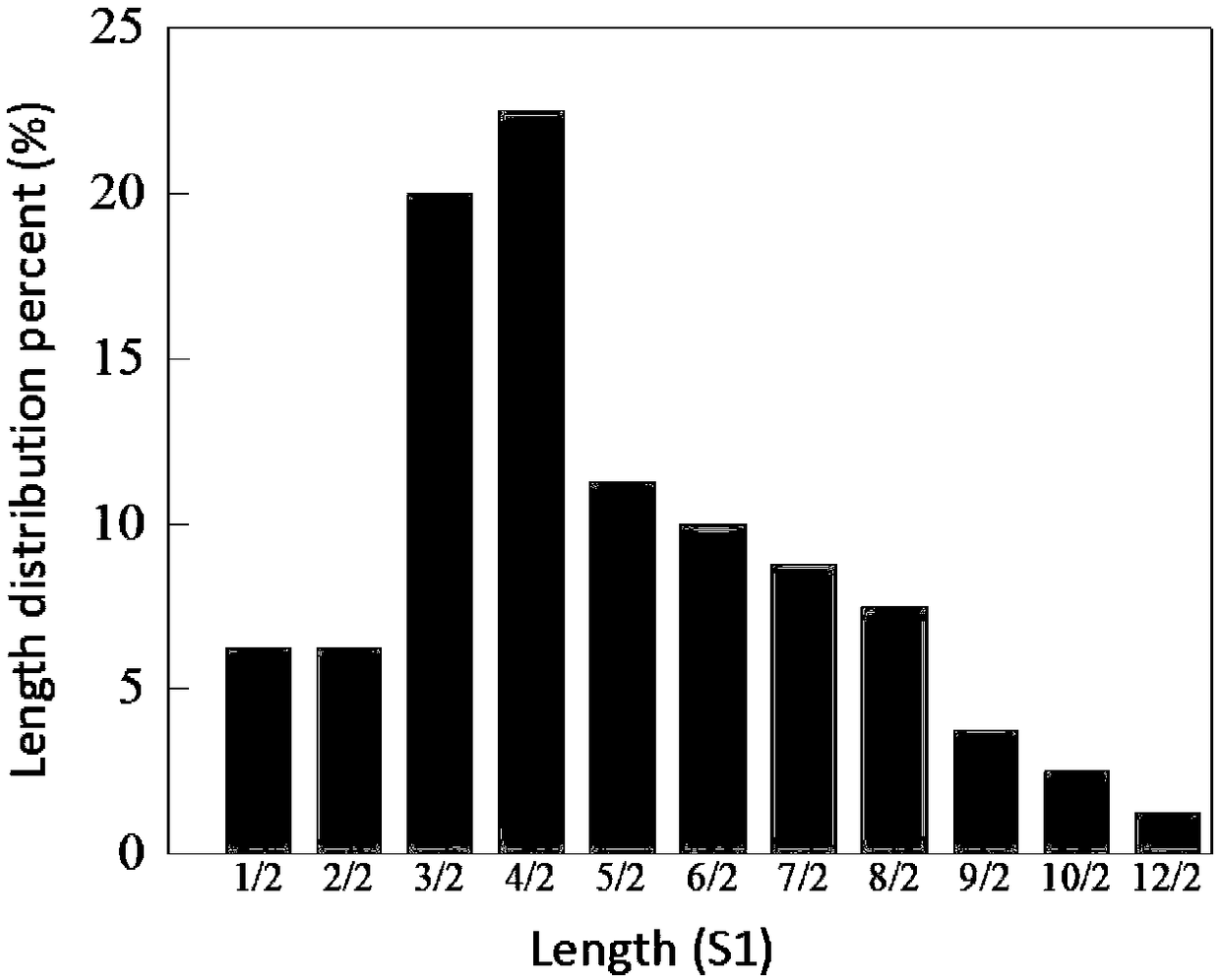
[0081] According to the amino acid sequence of the parent short peptide 9S1 (AEAEAKAKAEAEAKAK) 9 , chemically synthesize its gene and connect it to the N-terminal of the gene sequence of the enzyme to be screened (PGL, LOX and ASN), and clone it into the plasmid pET-22b(+) / enzyme of the target enzyme or protein (enzyme is the protein to be screened The expression gene, here PGL, LOX and ASN) between the NdeI and NcoI restriction sites, was constructed as a pET-22b(+) / 9S1-enzyme plasmid. The GFP expression gene gfp was fused to the end of pET-22b(+) / 9S1-enzyme to construct pET-22b(+) / 9S1-enzyme-gfp plasmid.
[0082] Using pET-22b(+) / 9S1-enzyme-gfp plasmid as template, degenerate upstream primer nSAP-up and specific downstream primer nSAP-down enzyme The linearized gene fragments of pET-22b(+) / nSAP-enzyme-gfp containing different amino acid compositions and lengths of SAP were obtained by PCR.
[0083] The ...
Embodiment 2
[0084] Embodiment 2: initial screening
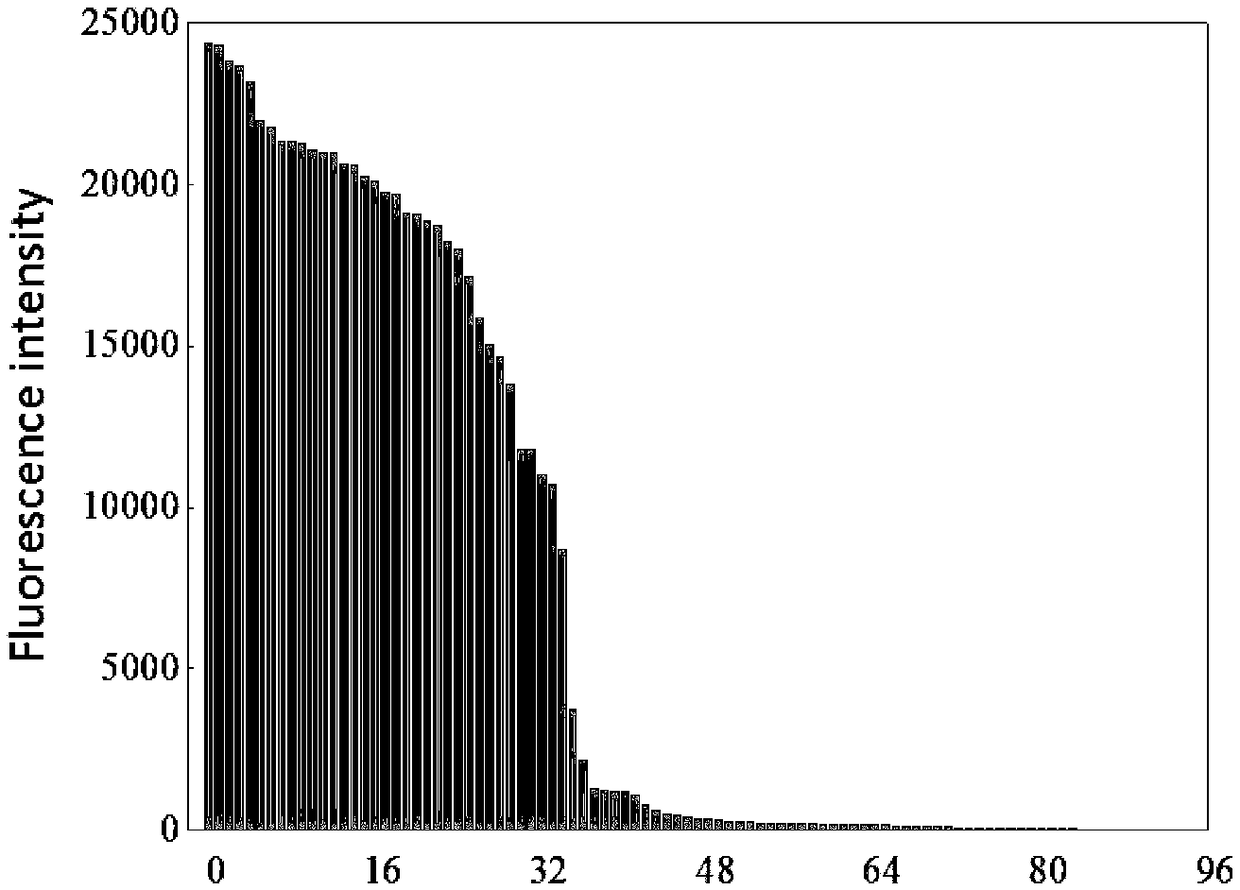
[0085] Primary screening: transform the recombinant plasmid and the recombinant plasmid library into the expression host Escherichia coli E.coliBL21(DE3), cultivate and induce in the seed medium, and continue to cultivate for a certain period of time (related to the expression of the enzyme to be screened), (related bacteria culture The method is: when OD 600When reaching 0.6, add IPTG induction (wherein the IPTG induction amount of PGL-GFP fusion enzyme is 0.04mM, and LOX-GFP is 1mM, and ASN-GFP is 1mM), and simultaneously adjusts temperature to cultivate under the most suitable induction temperature of this enzyme ( PGL-GFP was cultured at 30°C for 5h, LOX-GFP was cultured at 20°C for 10h, and ASN-GFP was cultured at 30°C for 5h). ) to dilute the cells to be screened, set the flow cytometer MoFlo XDP flow cytometry (Beckman Coulter, USA) nozzle size to 100 μm, 20 mM phosphate buffer of pH 7.4 as sheath fluid, cell OD 600 The detecti...
Embodiment 3
[0086] Embodiment 3: double screening identification
[0087] Re-screening and identification: The mutants with strong fluorescence intensity obtained after sorting by flow cytometry were cultured in shake flasks and measured for fluorescence intensity. Inoculate a single colony on the seed medium for overnight culture and then transfer to the fermentation medium, when OD 600 When reaching 0.6, add IPTG induction (wherein the IPTG induction amount of PGL-GFP fusion enzyme is 0.04mM, and LOX-GFP is 1mM, and ASN-GFP is 1mM), and simultaneously adjusts temperature to cultivate under the most suitable induction temperature of this enzyme ( PGL-GFP was cultured at 30°C for 48h, LOX-GFP was cultured at 20°C for 72h, and ASN-GFP was cultured at 30°C for 24h).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com