Drug preparation for treating chronic diabetic ulcers and preparation method thereof
A pharmaceutical preparation, a technology for chronic ulcers, applied in the field of medicine, can solve problems such as inability to achieve effects, and achieve the effects of inhibiting the production of inflammation, improving the curative effect, and promoting migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
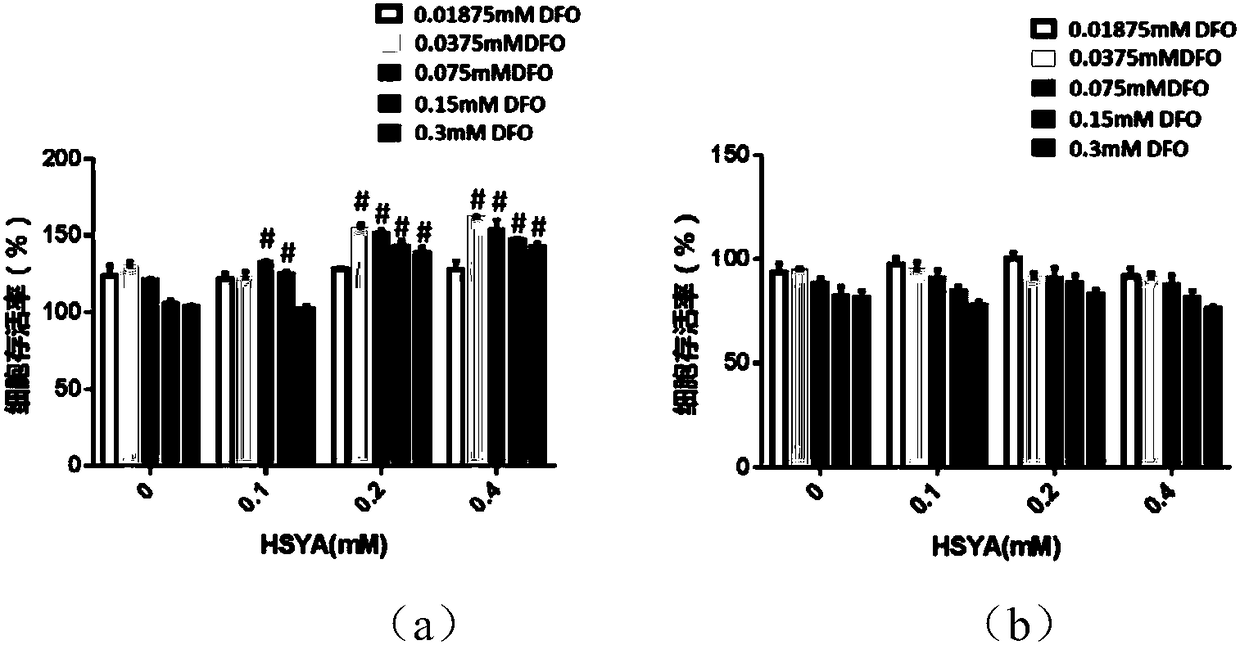
[0056] Example 1: Cytotoxicity Test (HSF)
[0057] 1. Culture skin fibroblasts in DMEM high-sugar medium (containing 10% inactivated fetal bovine serum, 1% double antibody (penicillin+streptomycin)), after the cells are confluent, digest the cells with trypsin, After gently tapping, add culture medium to stop digestion, carefully collect cells in a centrifuge tube, centrifuge, remove supernatant, resuspend cells and count. Adjust the cell suspension concentration to 2×10 4 cell / ml, and then add 200ul of cell suspension to each well of the 96-well plate (the edge wells are filled with sterile PBs). 5%CO 2 , incubated at 37°C, and adhered to the wall overnight until the cell monolayer covered the bottom of the well. Remove the supernatant, add HSYA (0.1, 0.2, 0.4mM), DFO (0.01875, 0.0375, 0.075, 0.15, 0.3mM) and their mixed solution with gradient concentration, 200ul per well, and set 5 duplicate wells for each concentration. At the same time, a blank control group (200ul cu...
Embodiment 2
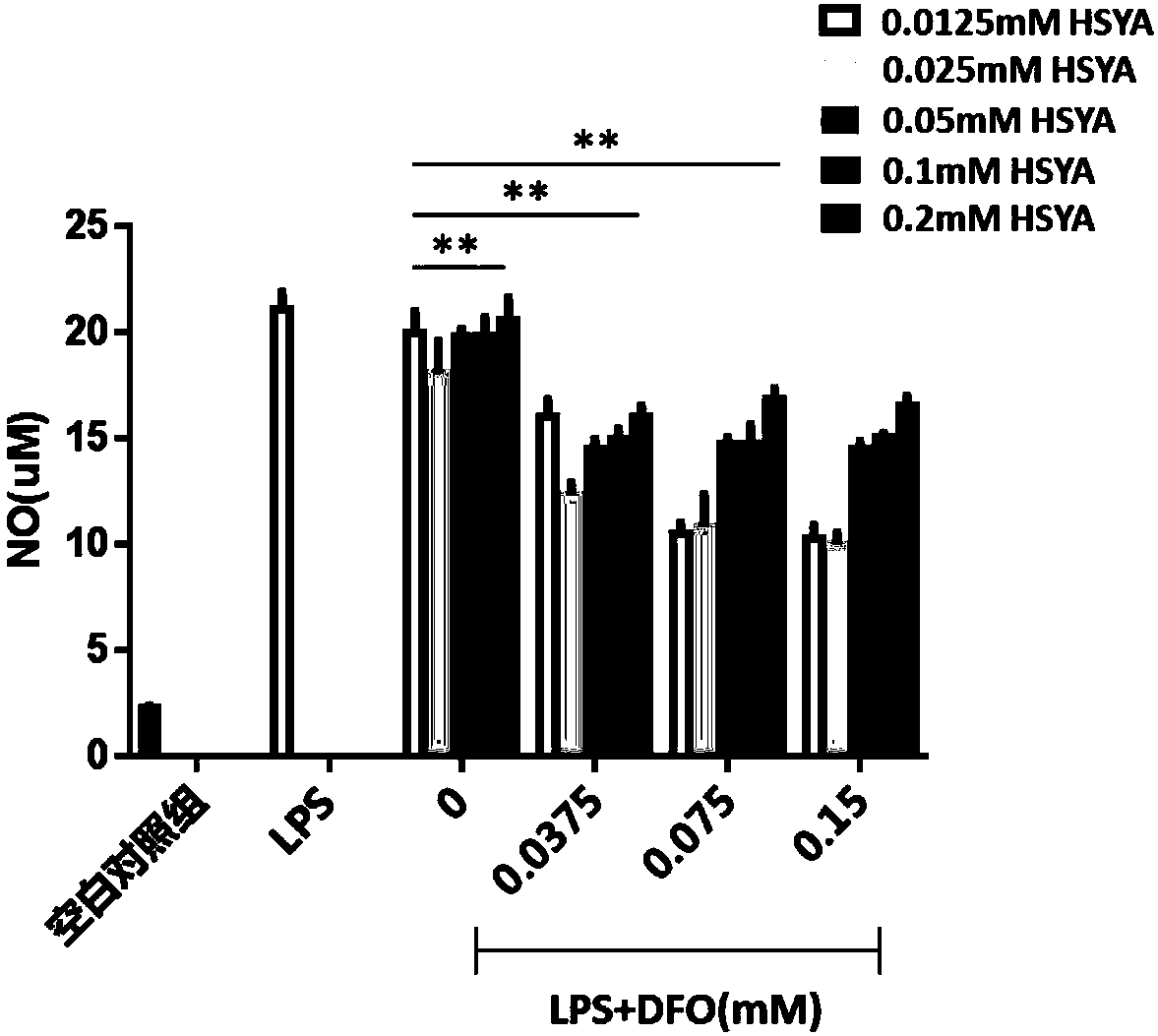
[0063] Embodiment 2: Anti-inflammatory experiment
[0064] 1. Culture the mononuclear macrophage cell line Raw264.7 in DMEM high-glucose medium (containing 10% inactivated fetal bovine serum, 1% double antibody (penicillin+streptomycin)), after the cells are confluent, use Digest the cells with trypsin, beat gently and add culture medium to stop the digestion, carefully collect the cells in a centrifuge tube, centrifuge, remove the supernatant, resuspend the cells to adjust the cell density to 5×10 5 cell / ml, Raw264.7 was inoculated in a 96-well plate by adding 0.2ml of cell suspension to each well (the edge wells were filled with sterile PBS). After culturing, add HSYA (0.0125, 0.025, 0.05, 0.1, 0.2mM), DFO (0.0375, 0.075, 0.15) and their mixed solution for pre-incubation for 4 hours, then add 10ul of LPS with a concentration of 1ug / ml in 96 wells plate, 5% CO 2 , after culturing at 37°C for 24h, the supernatant was collected for the detection of NO content, and all experim...
Embodiment 3
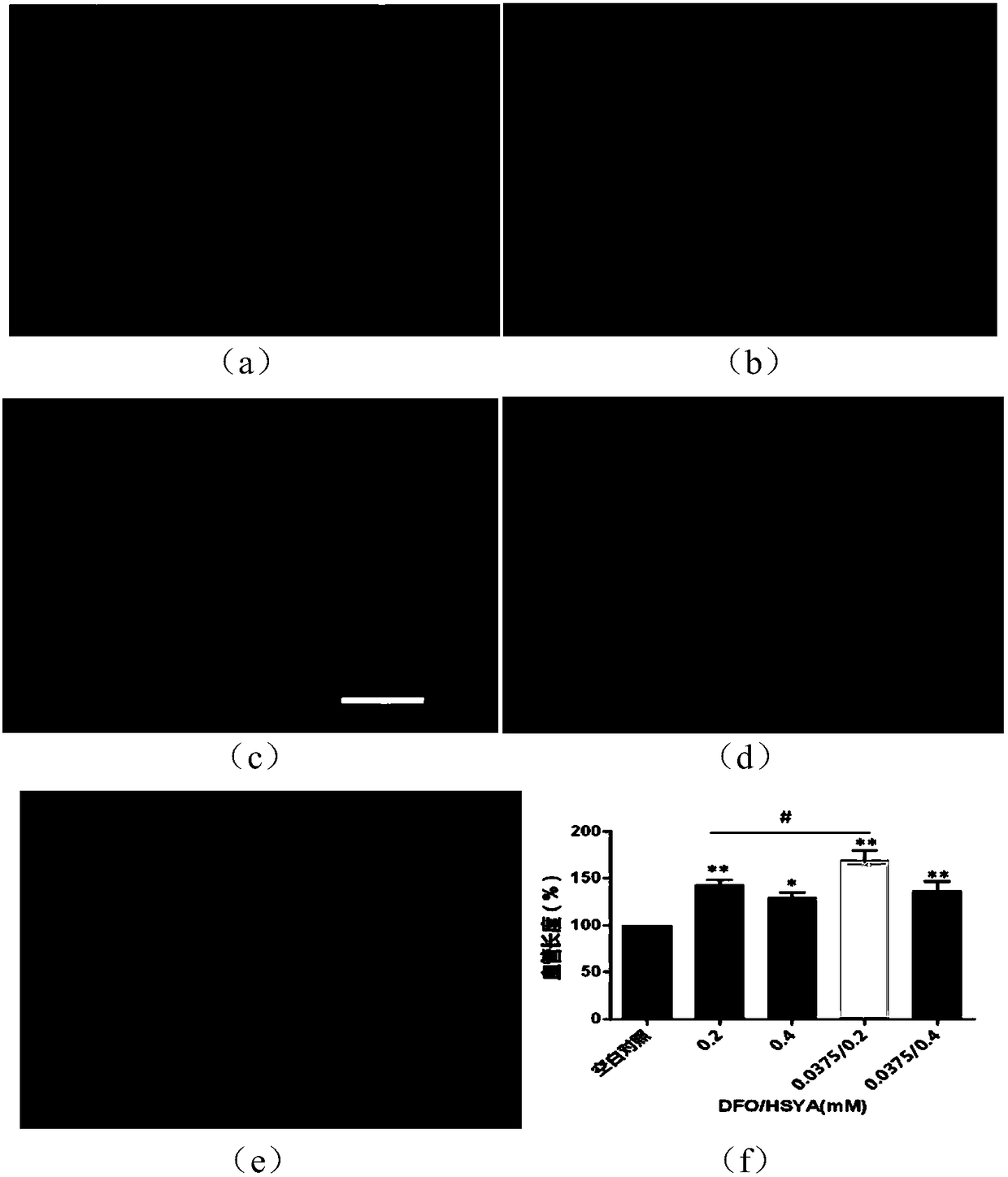
[0069] Example 3: Angiogenesis experiment
[0070] 1. Thaw Matrigel in a refrigerator at 4°C. After it becomes fluid, take 50ul of Matrigel and add it to the well of a 96-well plate, and place it in a 37°C incubator for 30min. Vascular endothelial cells were digested with trypsin, tapped gently, and then culture medium was added to stop the digestion. The cells were carefully collected in a centrifuge tube, centrifuged, the supernatant was removed, and the cells were resuspended and counted. Adjust the cell suspension concentration to 1.5×10 5 cell / ml, add 100ul cell suspension to each well, then add HSYA solution (0.2, 0.4mM), DFO (0.0375) and the mixed solution of the two, 5% CO 2 , and incubated at 37°C. After 6 hours, the 96-well plate was taken out, and 2.5uM calcein was added to incubate for 30 minutes, and then the formation of blood vessels was observed under an inverted fluorescence microscope. Three replicate wells were used for each concentration.
[0071] 2. Exp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com