Monoamine oxidase and gene and application thereof
A technology of monoamine oxidase and gene, applied in application, genetic engineering, plant genetic improvement, etc., can solve rare and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1. Acquisition of Monoamine Oxidase Encoding Gene
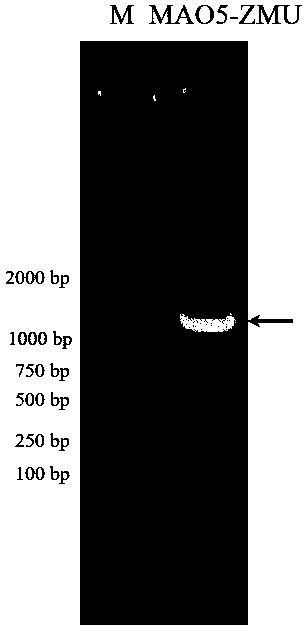
[0095] By analyzing the genome sequence of Pseudomonas strain, a 1248bp monoamine oxidase sequence was determined, a pair of specific primers were designed to amplify by PCR technology, and finally the full-length coding frame sequence of the gene was obtained. The specific method is as follows: using the extracted genomic DNA as a template, using primers 1: 5'-CCATGGGCCGTATAGCAATCATCG-3' and 2: 5'-CTCGAGTCACAGGTGCTCTCCGAAATG-3', and introducing restriction sites for PCR amplification. The PCR reaction system is as follows: 12.5 μL of 2×TaqPCR Master Mix, 1 μL of genomic DNA, 0.5 μL of upstream and downstream primers, ddH 2 O 10.5 μL. PCR reaction conditions: pre-denaturation at 95°C for 10 min; denaturation at 98°C for 10 s, annealing at 56°C for 30 s, extension at 72°C for 90 s as a cycle; extension at 72°C for 10 min, 30 cycles. The PCR product was electrophoresed on a 0.9% agarose gel to verify whether a ...
Embodiment 2
[0096] Example 2. Construction of monoamine oxidase-encoding gene recombinant expression vector
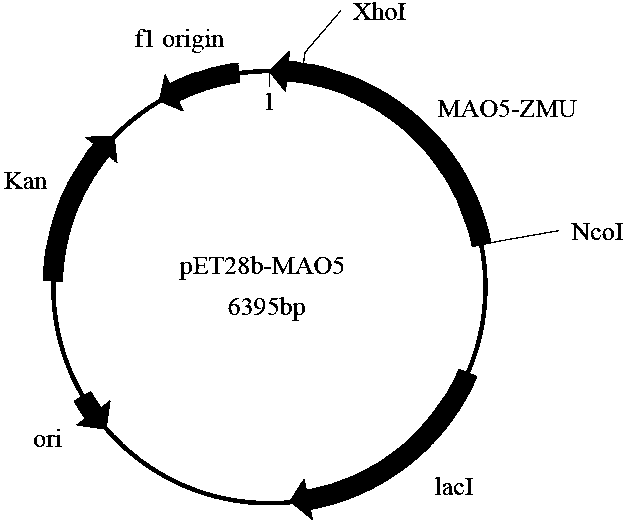
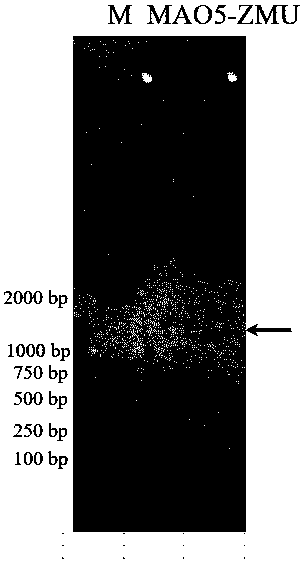
[0097] Using the plasmid pET28b(+) as the expression vector of the enzyme protein, a prokaryotic recombinant expression vector of the monoamine oxidase gene MAO5 was constructed. The specific method is as follows: extract the recombinant plasmid pGEM-T-MAO5, use restriction endonuclease NCOI / wxya Treat the recombinant plasmid, and use T4 DNA ligase (NEB) to connect the gene fragment with the expression vector pET28b(+) treated with the same restriction endonuclease to construct the expression vector pET28b(+)-MAO5. The constructed intracellular expression vector pET28b(+)-MAO5 was transformed into Escherichia coli BL21(DE3) by the heat shock method, spread on a plate containing kanamycin resistance, cultured at 37°C overnight, and clones were randomly selected for Colony PCR identification.
Embodiment 3
[0098] Example 3. Recombinant expression and detection of monoamine oxidase protein
[0099] The recombinant Escherichia coli BL21(DE3) / pET28b-MAO5 containing the intracellular expression recombinant plasmid pET28b-MAO5 after identification in Example 2 was respectively treated with LB liquid medium containing 50 μg / ml kanamycin resistance at 37°C, Cultivate at 250rpm for 12h, then inoculate with 1% inoculum (v / v) into fresh LB liquid medium containing 50μg / ml kanamycin resistance, and cultivate at 37˚C and 250rpm to the cell concentration OD 600About 0.6, then add IPTG with a final concentration of 0.01mM to the LB liquid medium, induce culture at 20°C, 250rpm for 20h, centrifuge at 4°C, 8000rpm for 5min, and collect the bacterial cells containing recombinant monoamine oxidase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com