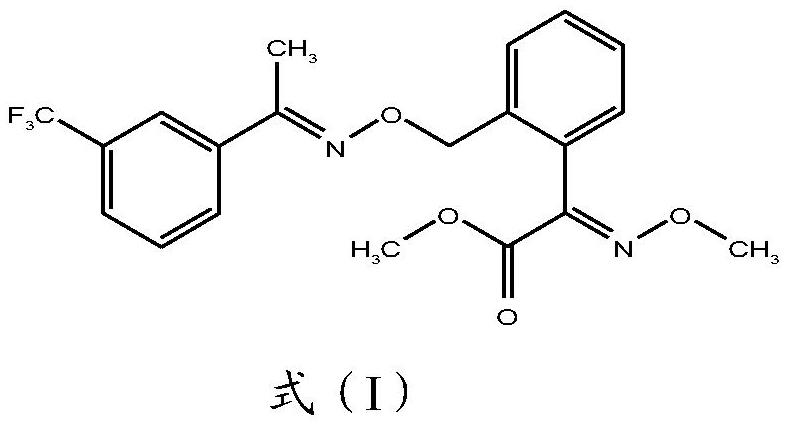
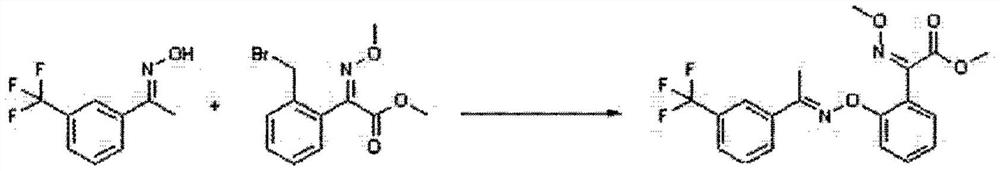
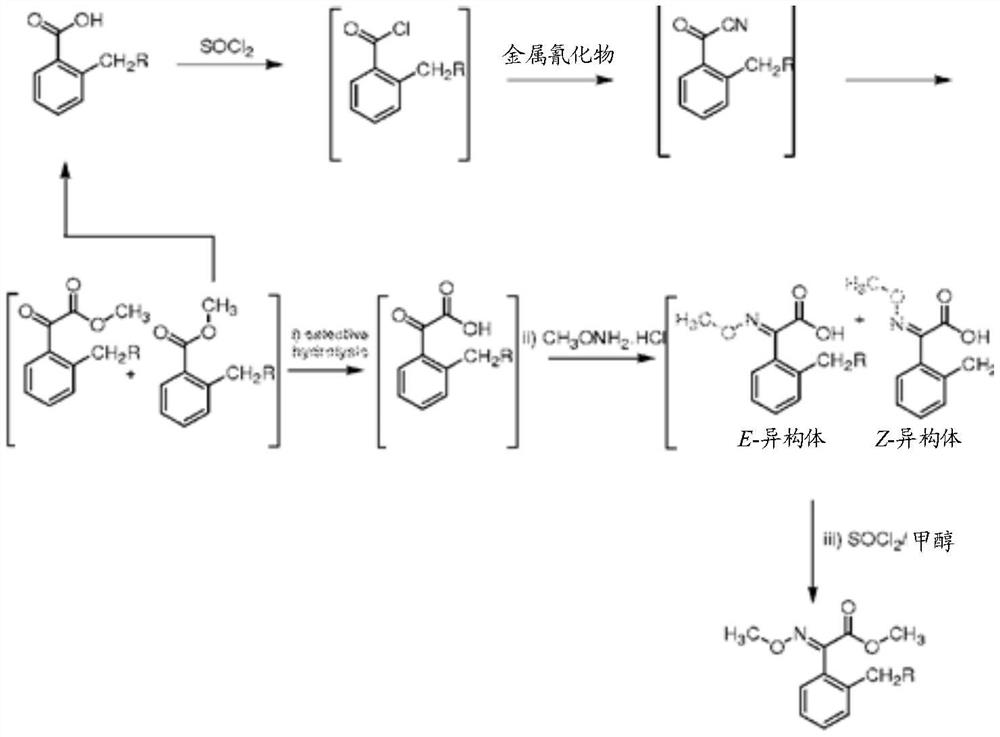
A kind of method for preparing trifloxystrobin
A technology of methyl acetate and compounds, applied in the field of preparation of trifloxystrobin, which can solve the problems affecting the overall process cost and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Preparation of Hydroxy-O-Methoxy-Acetonitrile (Method A)
[0085]
[0086] 2-Methylbenzaldehyde (120.15gm 1.0mol) was slowly added to the four-neck solution containing water (125mL) and sodium cyanide 49.00gm (1.0mol) at a temperature of 8°C to 10°C under stirring within 0.5 hours in the flask. The solution was prepared by adding sodium cyanide 49.00 gm (1 mol) dropwise to water (125 mL), the mixture was stirred over 30 minutes, during which time the temperature was maintained at -5°C to 5°C, and then the temperature was raised to 8°C ~10°C. After the addition was complete, the reaction mass was stirred for 15 minutes, then 40% sulfuric acid [83 gm sulfuric acid (0.84 mol) in 125 ml of water] was added within three hours, the temperature was maintained between 10° C. and 20° C. and the reaction mixture was stirred for 15 minutes. minute. After the reaction was completed, the product was extracted with dichloroethane and dried over anhydrous sodium sulf...
Embodiment 2
[0087] Example 2: Preparation of Hydroxy-O-Methoxy-Acetonitrile (Method B)
[0088] 2-Methylbenzaldehyde (24.03 gm, 0.2 mol) was added to a solution of concentrated hydrochloric acid (18 ml, 21.6 g, and 0.2 mol) and water (17 ml) in a 200 ml three-necked flask at 0°C. The resulting mixture was cooled to -5°C to yield a slurry of 2-methylbenzaldehyde hydrochloride. Sodium cyanide solution (11.00 g, 0.22 mol) in 40 g of water was added to the reaction mixture dropwise and the mixture was stirred over 30 minutes during which time the temperature was maintained at -5°C to -1°C. During the addition, a yellow solution was obtained after adding about half of the cyanide solution, but formed a slurry again toward the end of the addition. Stirring was continued for one hour after the addition was complete. Hydrochloric acid (1 ml) was added to bring the mixture to pH=7. After the reaction was completed, the product extracted with ethyl acetate was added, and the mixture was warmed...
Embodiment 3
[0089] Example 3: Preparation of Hydroxy-O-Methoxy-Acetonitrile (Method C)
[0090] 2-Methylbenzaldehyde (120.15 gm 1.0 mol) was added to a solution of sodium bisulfite (105 gm 1.0 mol) in water (475 gm) at room temperature, and the temperature was kept below 35°C. Sodium cyanide (49 gm 1.0 mol) was added to the solution in portions, and the temperature was maintained between 25°C and 35°C. The oil phase separates out as the upper layer, and it separates from the lower aqueous layer. The aqueous layer was then extracted with dichloroethane, and the resulting dichloroethane extract was combined with the separated oil layer. The organic layer was dried over anhydrous sodium sulfate, and subjected to vacuum distillation in a hot bath at 40°C to remove dichloroethane. The amount of hydroxy(2-tolyl)acetonitrile obtained remaining was 132 g. The yield of hydroxy(2-tolyl)acetonitrile was 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com