Limonoids for treating autoimmune diseases
An autoimmune disease, limonin technology, applied in allergic diseases, metabolic diseases, skin diseases, etc., can solve problems such as limited treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
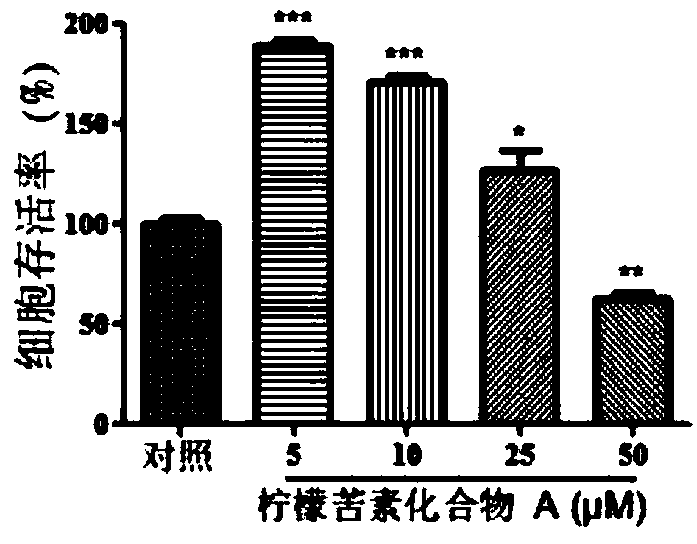
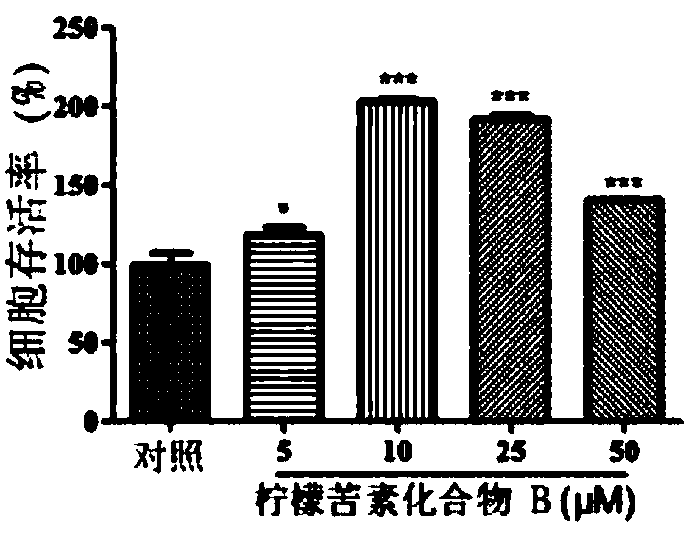
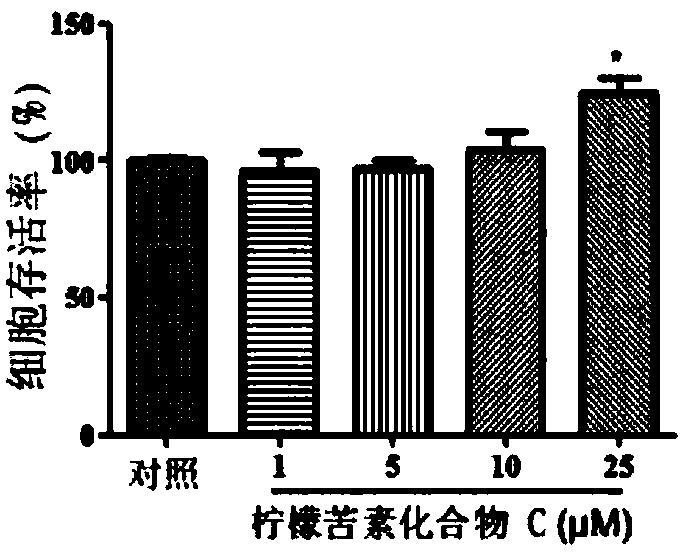
[0123] Purpose:
[0124] In this experiment, the effects of limonin compound A, limonin compound B, limonin compound C and limonin compound D on the cell survival rate of human peripheral blood mononuclear cells were determined.
[0125] method:
[0126] Human peripheral blood mononuclear cells were isolated from human blood buffy coat provided by Macau Blood Transfusion Center. Specifically, the human blood buffy coat and normal saline were diluted in a 1:1 ratio in a 50 ml centrifuge tube, and then peripheral blood mononuclear cells were obtained by Ficoll density gradient centrifugation. Human peripheral blood mononuclear cells were collected, washed twice with saline and centrifuged at 350g for 10 minutes. These cells can be used for subsequent experiments.
[0127] Cell viability experiment:
[0128] Human peripheral blood mononuclear cells were cultured in RPMI 1640 medium (containing 10% fetal bovine serum (by volume), penicillin (100 units / ml) and streptomycin (100...
Embodiment 2
[0132] Purpose:
[0133] In this experiment, the effects of limonin compound A, limonin compound B, limonin compound C and limonin compound D on ROR-γt luciferase activity were determined.
[0134] method:
[0135] Lightswitch luciferase reporter assay, ROR Gamma T LUCPorterTM Stable Reportercells and LUCPorterTM Vector Control HEK293 cells were cultured in RPMI 1640 medium (containing 10% fetal bovine serum (volume ratio), penicillin (100 units / ml) and streptomycin (100 g / mL) and puromycin (3 g / mL)). They are divided into 5×10 4 cells per well in a 96-well plate. Then 5, 10, 25 and 50 μM of limonin compound A or limonin compound B or limonin compound C or limonin compound D or 10 μM of positive control drug digoxin were added. Incubate in a 37°C incubator for 16 hours, then add the luciferase assay reagent to a 96-well plate, and react in the dark at room temperature for 30 minutes, then transfer the liquid to a white 96-well plate, and detect the fluorescence value with ...
Embodiment 3
[0139] Purpose:
[0140] In this experiment, the effects of limonin compound A, limonin compound B, limonin compound C and limonin compound D on the expression of ROR-γt protein in HEK293 cells were determined.
[0141] method:
[0142] Transfection of ROR-γt plasmid into HEK293 cells
[0143] Transfection experiments were performed according to the instructions of Lipofectamine LTX transfection reagent. HEK293 cells were purchased from American ATCC Company, and the cells were cultured in DMEM medium (containing 10% fetal bovine serum (volume ratio), penicillin (100 units / ml) and streptomycin (100 g / ml)). Take 5×10 5 The cells were seeded in 6-well plates, and 1.5 ml of DMEM medium was added to them for later use. Then 500 microliters of opti-MEM medium was added with 1.25 micrograms of ROR-γt plasmid, plus 1.25 microliters of PLUS reagent, mixed gently, and reacted at room temperature for 5 minutes. Then add LTX reagent, mix gently, and react at room temperature for 30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com