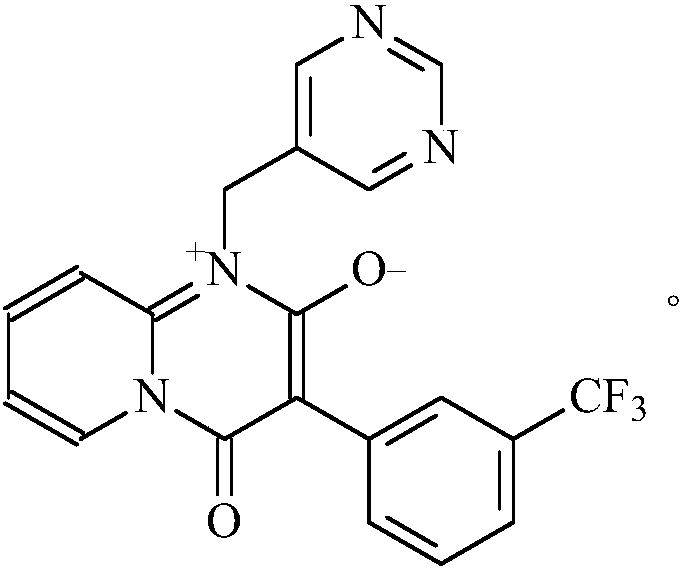
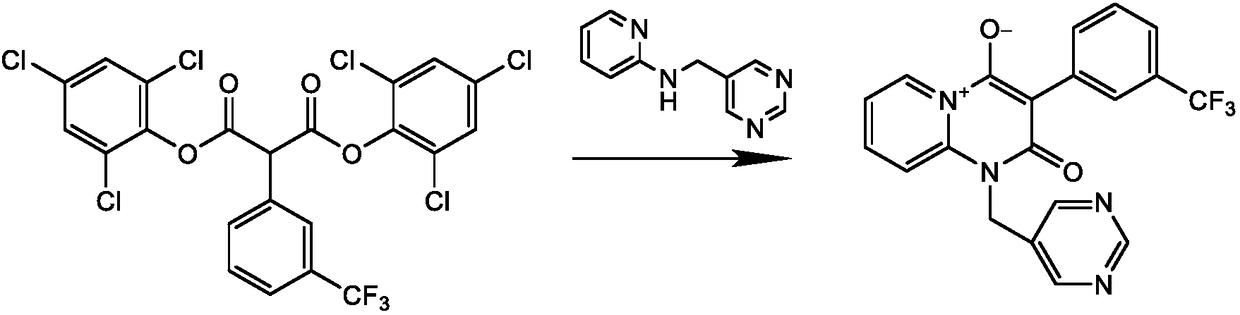
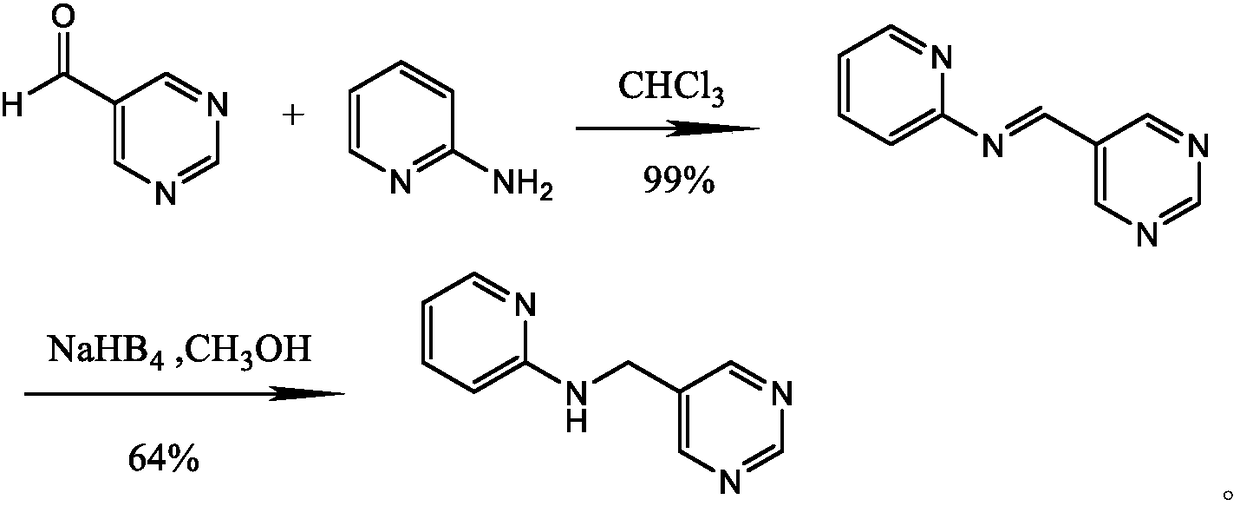
Preparation method of N-2-pyridine-5-pyrimidine methylamine
A technology for pyrimidine methylamine and pyridine, which is applied in the field of preparation of N-2-pyridine-5-pyrimidine methylamine, can solve the problems of difficult to obtain, expensive sodium borohydride, low reduction yield of sodium borohydride and the like, and achieves a reduction in the The effect of high cost, high yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of N-2-pyridine-5-pyrimidinemethylamine, comprising the steps of:
[0038] (1) Synthesis of 3-amino-2-methacrolein: 114g (1.0mol) 3-ethoxy-2-alkylacrolein was added to a 500ml reaction flask with a stirrer and a thermometer, and used in ice / Cool with brine, add 750ml of 25% ammonia water dropwise at 0-10°C, dropwise for 1-2 hours, after the dropwise addition, slowly heat up to 25°C, the solution is yellow, continue to react for 2-3 hours; then concentrate the reaction under reduced pressure The mixture, 3-aminoacrolein crystals precipitated, was recrystallized from ethyl acetate to obtain 76.5 solids, the yield was 90%, and the melting point was 113-114°C;
[0039] (2) Synthesis of 5-methylpyrimidine: Add 85.0g (1.0mol) 3-amino-2-alkylacrolein, 54g (1.2mol) formamide and 2.0g piperidine acetate with a stirrer, reflux Put the condenser tube and thermometer in a 500ml reaction bottle, raise the temperature to 125°C, continue the reaction for 12-14h;...
Embodiment 2
[0043] A preparation method of N-2-pyridine-5-pyrimidinemethylamine, comprising the steps of:
[0044] (1) with embodiment 1;
[0045] (2) with embodiment 1;
[0046](3) Synthesis of 5-bromomethylpyrimine: Dissolve 94.0g (1.0mol) of 5-methylpyridine in 500ml of carbon tetrachloride solution, add 2.g of 2,2-azoisobutyronitrile, heat Reflux, then add bromine 176g (1.1mol) dropwise, and continue the reaction for 2-4 hours after the addition; then cool to room temperature, filter, and the filtrate is washed with aqueous sodium sulfite, dried over magnesium sulfate, and concentrated. Distilled under reduced pressure, collected 65-66 ° C / 10mmHg fraction 111.8g, yield 65%, 1H NMR (CDCl3): δ4.40 (s, 2H), 8.79 (s, 2H), 9.16 (s, 1H).
[0047] (4) Synthesis of N-2-pyridine-5 pyrimidinemethylamine Synthesis: in 500mL equipped with stirrer, thermometer, condenser tube
[0048] In a four-necked reaction flask, add 137.6g (0.8mol) of 5-bromomethylpyrimidine, 77.1g (0.82mol) of 2-aminopy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com