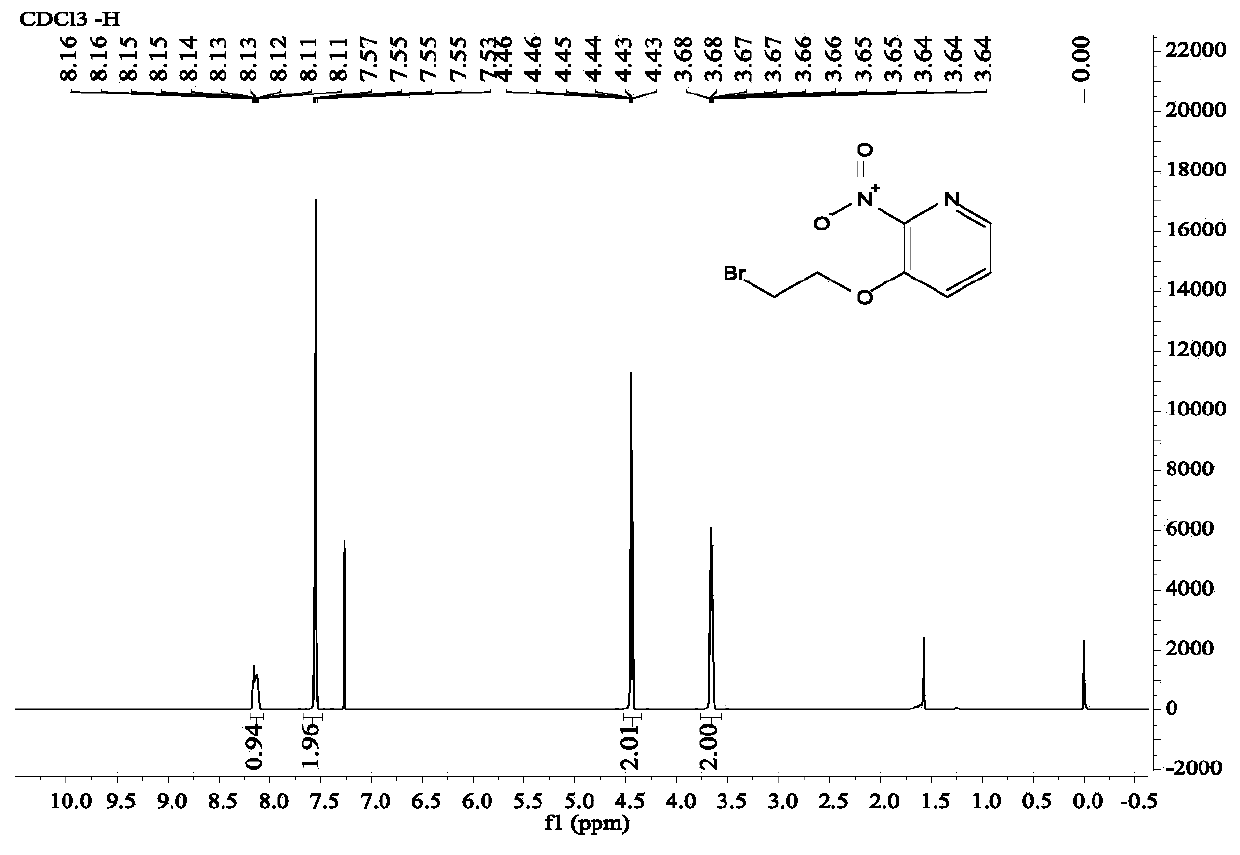
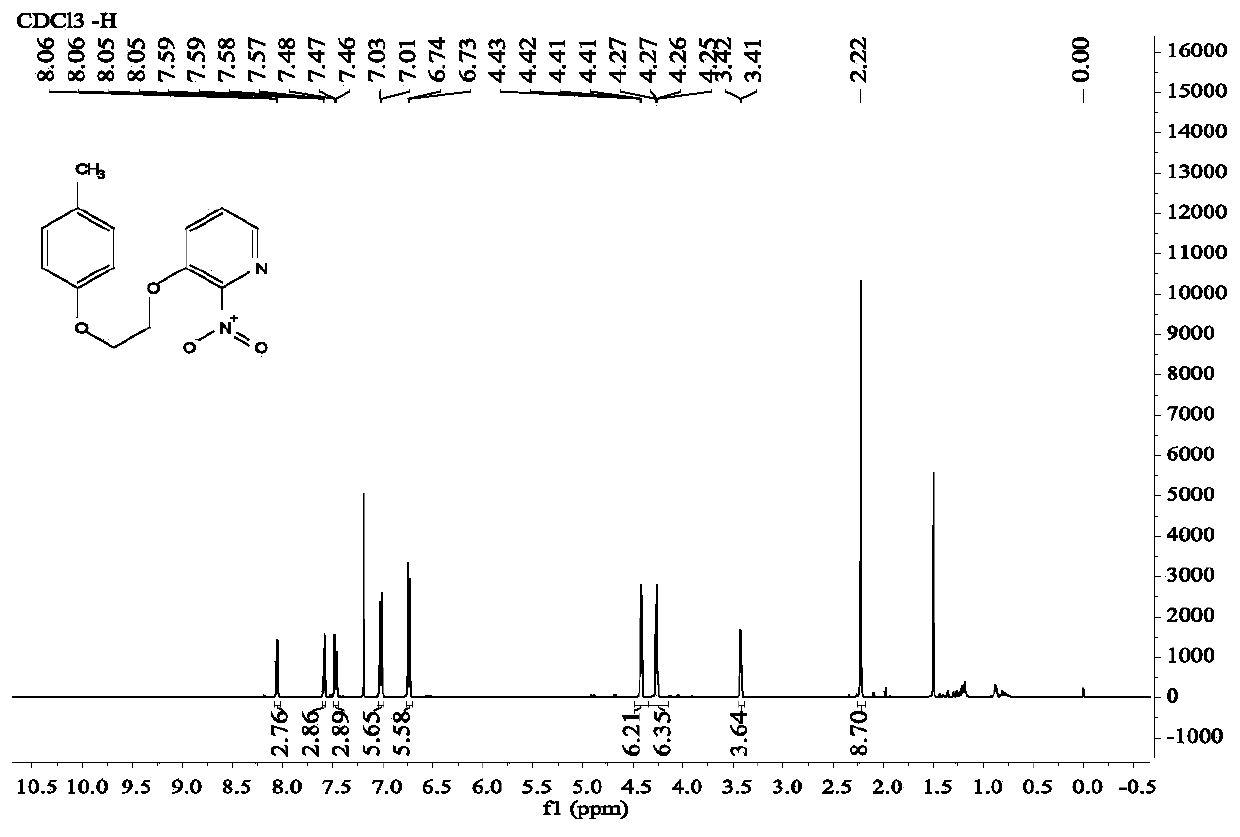
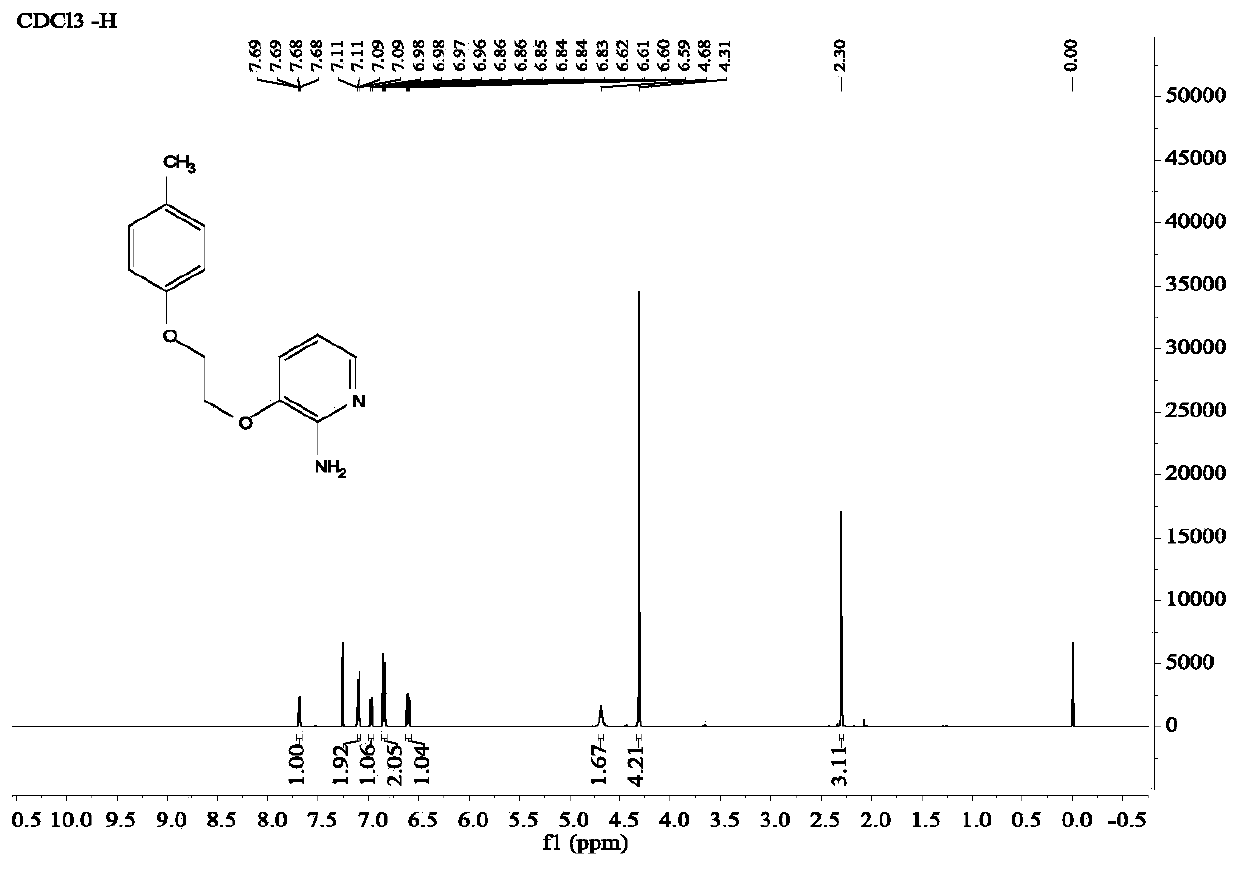
A kind of diaryl ethylene ether compound and its pharmaceutically acceptable salt, synthetic method
A technology of diaryl ethylene ether and synthetic method, which is applied in the fields of organic chemistry, antineoplastic drugs, and drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] The present invention and the manner in which the present invention is carried out are further described below in conjunction with the examples. These examples are only for further elucidating the present invention rather than limiting the protection of the present invention thereto.
[0043] The invention will now be further described with reference to the following illustrative examples, in which unless otherwise indicated:
[0044] (1) Temperatures are given in degrees Celsius, operating at room or ambient temperature.
[0045] (2) The organic solution was dried over anhydrous magnesium sulfate.
[0046] (3) The final product has satisfactory proton NMR and mass spectra.
[0047] (4) The yields given are for illustration only, and higher yields can be obtained for process development, and if more are needed, repeat the preparation.
[0048] (5) Column chromatographic purification was carried out using a self-packed silica gel column.
[0049] (6) A quadrupole orb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com