Lead iodide material with controllable shape and preparation method of lead iodide material
A lead iodide and morphology technology, applied in lead halide and other directions, can solve problems such as affecting the photoelectric conversion performance of perovskite materials, and achieve the effects of convenient operation, simple process and uniform size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Prepare the acetic acid solution of lead acetate: 1.14g (3mmol) lead acetate trihydrate is dissolved in acetic acid aqueous solution, and the mass fraction of acetic acid aqueous solution is 5%, stirs 10 minutes and fully dissolves, obtains 0.1mol / L lead acetate aqueous solution.
[0028] (2) Preparation of potassium iodide aqueous solution: Dissolve 0.5 (3 mmol) potassium iodide in 5 mL of deionized water, and ultrasonically dissolve it completely for 5 minutes to obtain potassium iodide solution.
[0029] (3)PbI 2 Preparation of materials: the potassium iodide solution in step (2) was added dropwise to the lead acetate solution in step (1), and stirred for 10 minutes after completion. Then the solution was transferred to a 50mL reactor, and placed at 120°C for 8 hours. Centrifuge and wash with deionized water until neutral, vacuum-dry at 80°C after vacuum filtration to obtain PbI 2 Material.
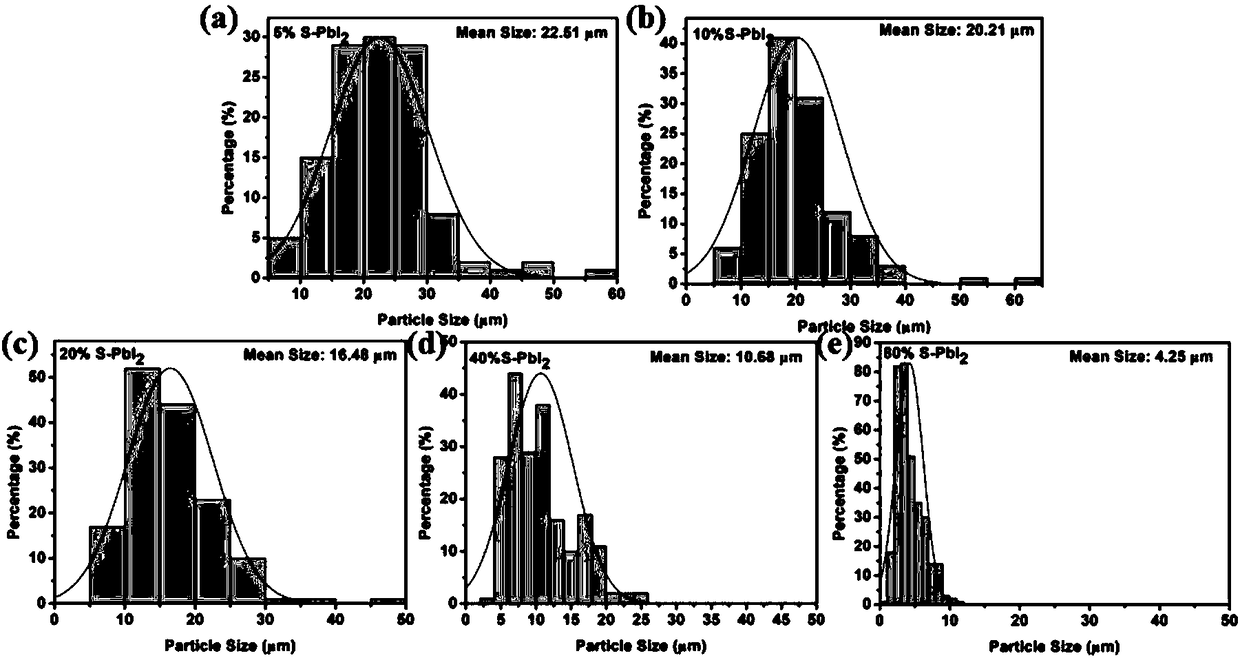
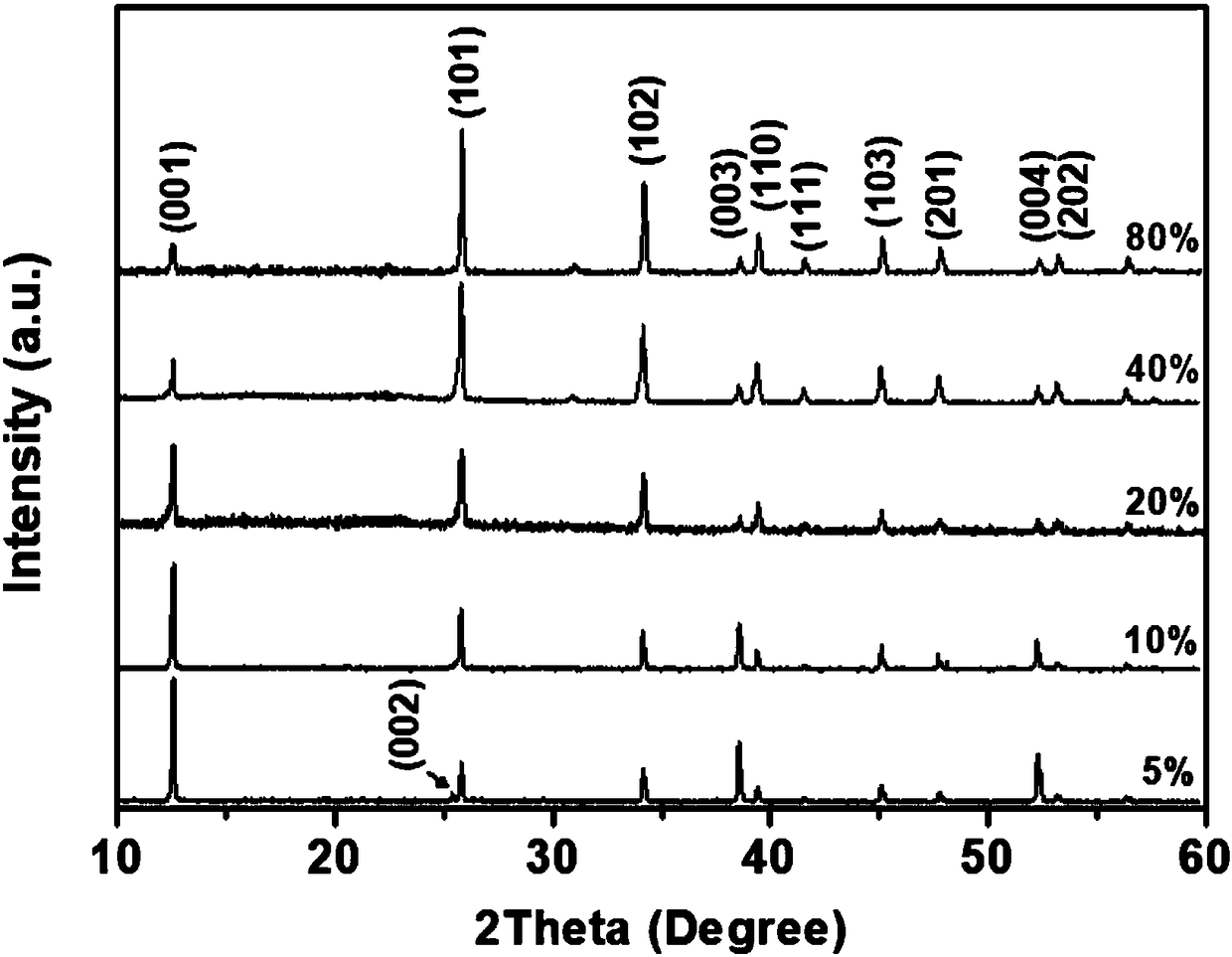
[0030] figure 1 Among (a) and (f) are the PbI prepared according to...
Embodiment 2
[0038] (1) Prepare the acetic acid solution of lead acetate: lead acetate trihydrate is dissolved in the acetic acid aqueous solution, the acetic acid aqueous solution mass fraction is 10%, stirs 10 minutes and fully dissolves, obtains the lead acetate aqueous solution, the concentration scope is in the 0.086mol / L scope.
[0039] (2) Preparation of potassium iodide aqueous solution: 0.5 g (3 mmol) of potassium iodide was dissolved in 5 mL of deionized water, and ultrasonically dissolved for 5 minutes to obtain a potassium iodide solution.
[0040] (3)PbI 2 Preparation of materials: the potassium iodide solution in step (2) was added dropwise to the lead acetate solution in step (1), and stirred for 10 minutes after completion. Then the solution was transferred to a 50mL reactor, and placed at 120°C for 8 hours. Centrifuge and wash with deionized water until neutral, vacuum filter and dry at 80°C to obtain PbI 2 Material.
[0041] figure 1 Middle (b) and (g) are the PbI pre...
Embodiment 3
[0045] (1) Prepare the acetic acid solution of lead acetate: lead acetate trihydrate is dissolved in the aqueous acetic acid solution, the mass fraction of the aqueous acetic acid solution is 20%, stir and dissolve fully for 10 minutes, obtain the aqueous lead acetate solution, the concentration range is within the scope of 0.12mol / L.
[0046] (2) Preparation of potassium iodide aqueous solution: 0.5 g (3 mmol) of potassium iodide was dissolved in 5 mL of deionized water, and ultrasonically dissolved for 5 minutes to obtain a potassium iodide solution.
[0047] (3)PbI 2 Preparation of materials: the potassium iodide solution in step (2) was added dropwise to the lead acetate solution in step (1), and stirred for 10 minutes after completion. Then the solution was transferred to a 50mL reactor, and placed at 120°C for 8 hours. Centrifuge and wash with deionized water until neutral, vacuum filter and dry at 80°C to obtain PbI 2 Material.
[0048] figure 1 Middle (c) and (h) a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size distribution | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com