Carotane sesquiterpene compound as well as preparation and application thereof
A technology of sesquiterpenes and carotene, which is applied in the field of carotane sesquiterpenes and its preparation, can solve the problems of non-red tide biological hazards, environmental secondary pollution, drug residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
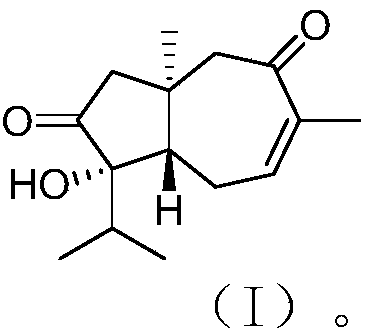
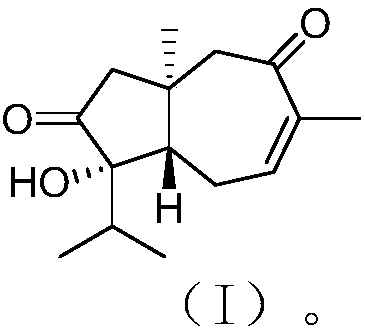
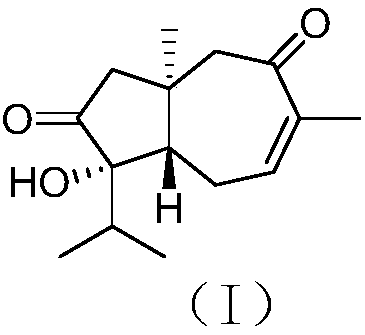
[0025] The structure of carotane sesquiterpenoids derived from algal epiphyte fungi is shown in formula (I).
[0026]
[0027] This compound has the following physicochemical and spectral properties:
[0028] Colorless oil; specific rotation [α] 20 D –114 (c 0.13, MeOH); H NMR spectrum (solvent is deuterated chloroform) δ H 2.29(d, 16.1), 2.25(d, 16.1), 2.41(dd, 12.0, 3.7), 2.61(m), 2.51(dddd, 20.1, 7.1, 3.7, 0.9), 6.43(dq, 7.1, 1.4), 2.84(d,15.7), 2.64(d,15.9), 1.78(heptet,6.9), 0.97(d,6.9), 0.91(d,6.8), 1.89(br s), 1.02(s); CNMR (solvent is deuterated chloroform) δ C 36.4(C), 54.1(CH 2 ), 218.5(C), 81.2(C), 48.6(CH), 28.4(CH 2 ), 139.3(CH), 137.3(C), 201.3(C), 57.8(CH 2 ),36.7(CH),16.8(CH 3 ), 17.2(CH 3 ), 22.2 (CH 3 ), 20.2 (CH 3 ); high resolution mass spectrometry [M] + m / z 250.1570, calculated 250.1569.
Embodiment 2
[0030] The preparation method of carotane sesquiterpenoids as shown in formula (I):
[0031] Take the well-grown Trichoderma virens Y13-3 strain on the plate, cut it into small pieces and inoculate it in the potato dextrose liquid medium, put 300 ml of culture medium in each 1 liter conical flask, a total of 200 bottles, room temperature After static fermentation for 30 days, it was extracted three times with ethyl acetate and concentrated under reduced pressure to obtain 27.2 g of crude extract after concentration.
[0032] The potato glucose liquid medium is composed of 500 ml of boiled juice containing 100 grams of potatoes per liter, 20 grams of glucose, 5 grams of peptone, 5 grams of yeast extract and 500 ml of Chenhai seawater.
[0033] Trichoderma virens Y13-3 strain was preserved in CCTCC, China Type Culture Collection Center on January 10, 2018, address: Wuhan University, China, preservation number is CCTCC NO: M 2018016, classified as Trichoderma virens, The strain ...
Embodiment 3
[0038] The difference from Example 2 is that
[0039] Take the well-grown Trichoderma virens Y13-3 strain on the plate, cut it into small pieces and inoculate it in Jerusalem artichoke glucose liquid medium, put 300 ml of culture medium in each 1 liter conical flask, a total of 100 bottles, room temperature The fermentation was static for 40 days, and the mycelium and fermentation broth were collected by filtration and separately.
[0040] The Jerusalem artichoke glucose liquid medium is composed of 500 ml of boiled juice containing 100 grams of Jerusalem artichoke tubers per liter, 20 grams of glucose, 5 grams of peptone, 5 grams of yeast extract, and 500 milliliters of old seawater.
[0041] About 30 liters of fermentation broth were collected, extracted three times with ethyl acetate, and concentrated under reduced pressure; the mycelium was dried and pulverized, and then extracted three times with ethyl acetate, and concentrated under reduced pressure; the concentrate was de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com