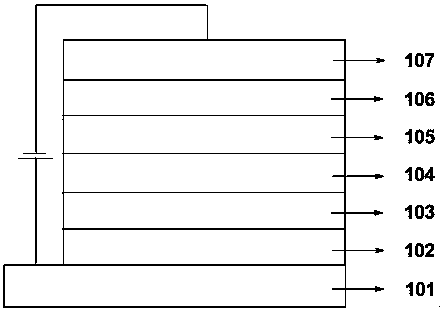
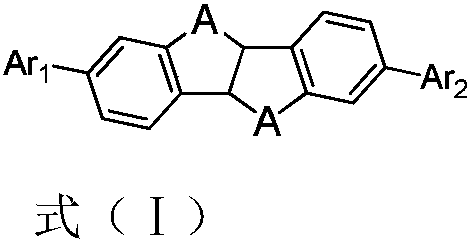
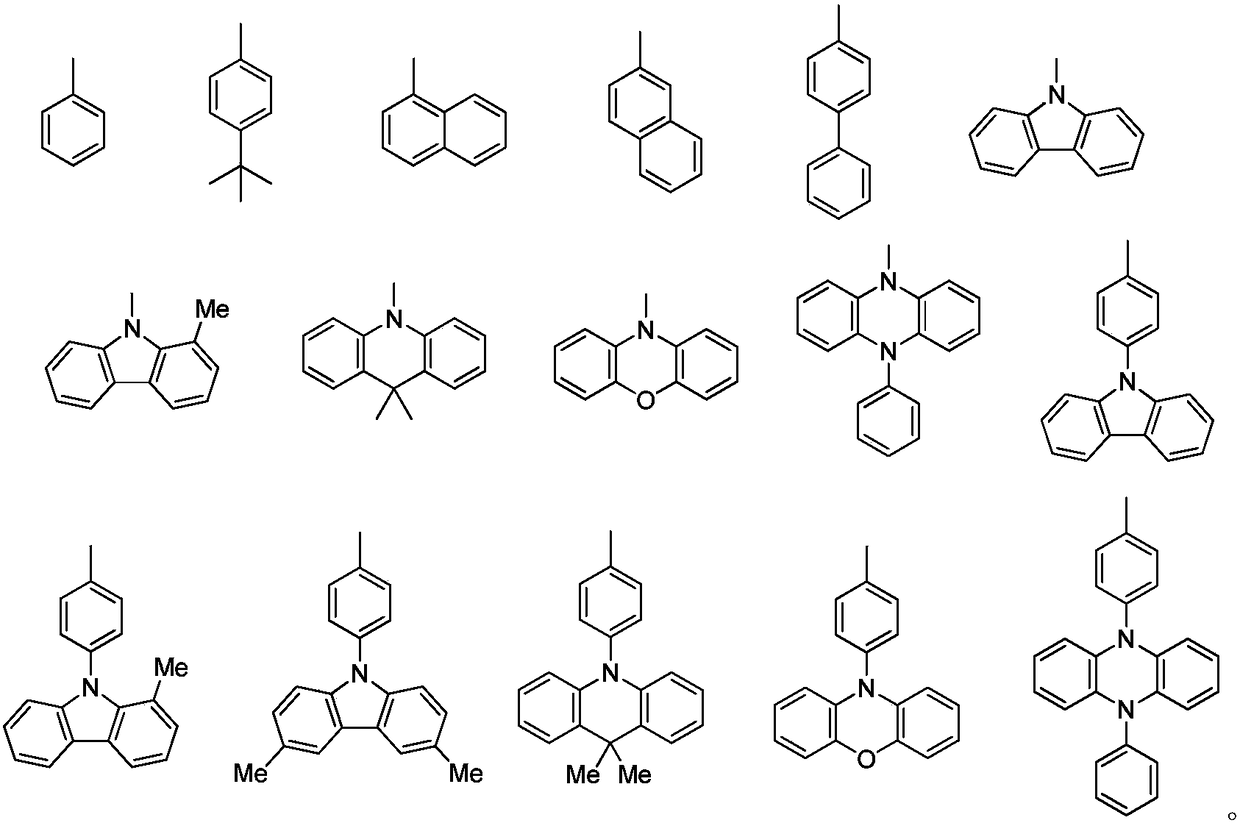
Thermally activated delayed fluorescent materials and application thereof
A technology of heat-activated delayed and fluorescent materials, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of limiting the application space of phosphorescent materials, expensive phosphorescent materials, etc., and achieve excellent film stability and molecular quality , Excellent effect of device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 compound C01:
[0036]
[0037] Preparation of intermediate B1: In a 500mL three-necked flask, add raw material A1 (6.0g, 0.015mol, CAS-RN: 74129-87-6), raw material 9,9-dimethylacridine (8.78g, 0.042mol) , copper powder (2.5g, 0.04mol), potassium carbonate (8.3g, 0.06mol), o-dichlorobenzene (180g), under the protection of nitrogen, heat up to 160°C, keep the temperature for 32 hours, cool down to 25°C, add tetrahydrofuran 120g, suction filtration, collect filtrate, remove solvent under reduced pressure, obtain B1 crude product 15.4g, the gained crude product is purified by silica gel column chromatography, eluent is n-hexane: dichloromethane=1:1 (v / v), Obtained 7.4g of B1 fine product, yield 75%, high resolution mass spectrum, positive ion mode, molecular formula C 44 h 36 N 2 S 2 , the theoretical value is 656.2320, and the test value is 656.2327.
[0038] Preparation of compound C01: In a 500mL three-necked flask, add compound ...
Embodiment 2
[0039] The preparation of embodiment 2 compound C02:
[0040]
[0041] Using phenoxazine as a raw material, refer to the method described in Example 1 to prepare compound C02, and obtain 1.7 g of the target object, high-resolution mass spectrum, positive ion mode, molecular formula C 38 h 24 N 2 o 6 S 2 , theoretical value 668.1076, test value 668.1071, elemental analysis (C 38 h 24 N 2 o 6 S 2 ), theoretical value C: 68.25, H: 3.62, N: 4.19, O: 14.35, S: 9.59, measured value C: 68.27, H: 3.66, N: 4.17, O: 14.30, S: 9.60.
Embodiment 3
[0042] The preparation of embodiment 3 compound C03:
[0043]
[0044] Using 5-phenyl-5,10-dihydrodiazaxanthene as a raw material, refer to the method described in Example 1 to prepare compound C03, and obtain 1.9 g of the target compound, high-resolution mass spectrometry, positive ion mode, molecular formula C 50 h 34 N 4 o 4 S 2 , theoretical value 818.2021, test value 818.2027, elemental analysis (C 50 h 34 N 4 o 4 S 2 ), theoretical value C: 73.33, H: 4.18, N: 6.84, O: 7.81, S: 7.83, measured value C: 73.36, H: 4.18, N: 6.83, O: 7.80, S: 7.83.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com