Brain tissue membrane protein extraction method
An extraction method and membrane protein technology, which is applied in the field of membrane protein extraction, can solve the problems of protein denaturation, loss of protease activity and channel activity, and cannot be restored, and achieve the effect of efficient extraction and simple system formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
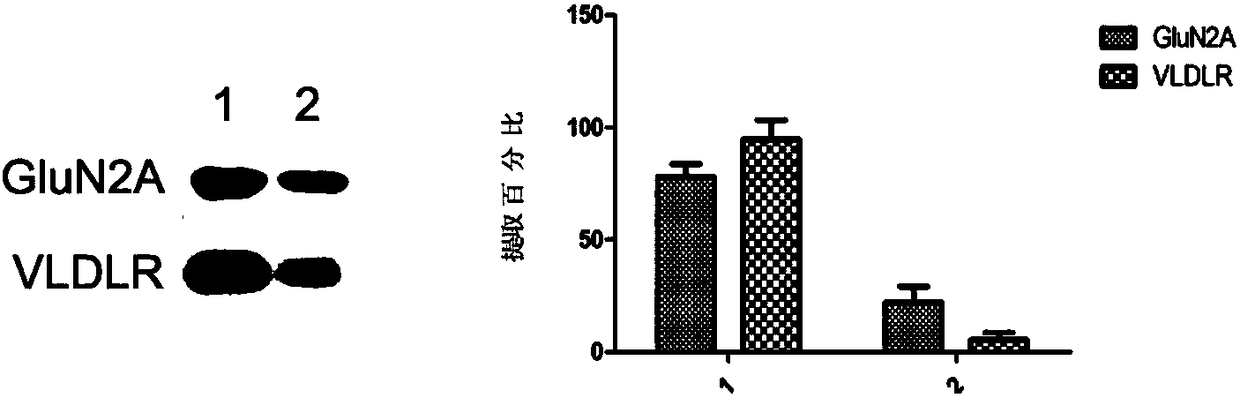
[0030] Such as figure 1 As shown, the present invention is used to extract two membrane-integrated proteins, namely GluN2A protein and VLDLR protein, and the undissolved precipitate is further lysed with a strong RIPA lysate to extract the unextracted protein of the present invention . The two parts of the protein were electrophoresed by SDS-PAGE in the same ratio, and it can be seen that more than 78% of GLuN2A and more than 95% of VLDLR were extracted by using the present invention.
Embodiment 2
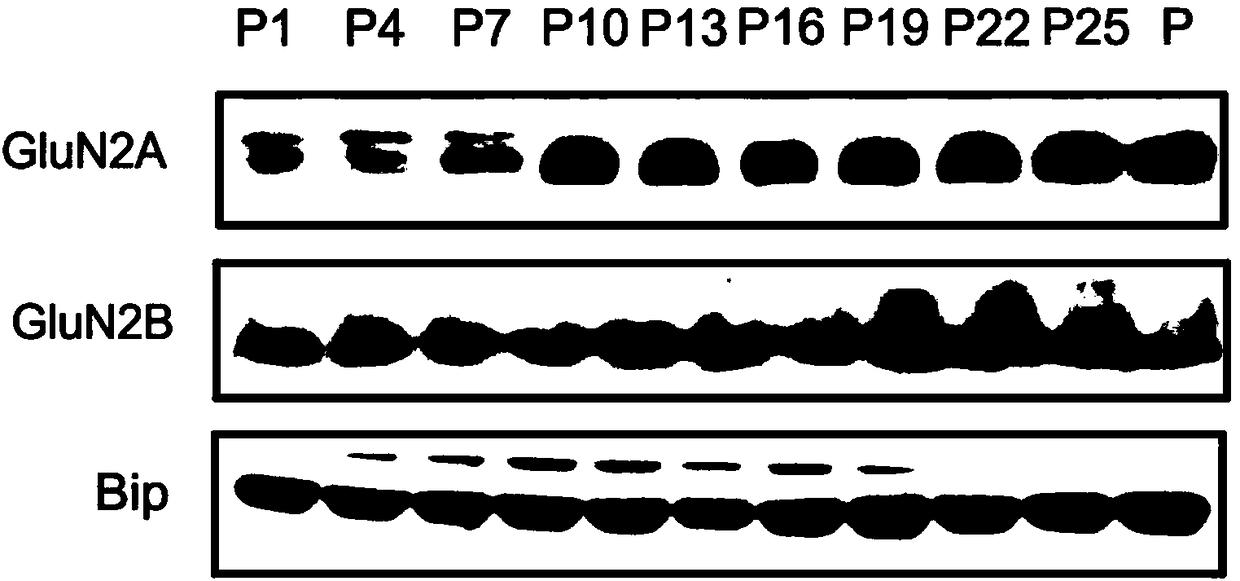
[0032] Using the present invention to extract membrane protein expression in mouse brain tissue at different stages of development and analyze:
[0033] 1. Take mice of different ages, namely mice and female mice born at 1, 4, 7, 10, 13, 16, 19, 22, and 25 days old.
[0034] 2. The mice were killed by neck dislocation, the brain tissue was quickly removed, and the cortex was separated.
[0035] 3. Weigh the isolated cortical tissue, add 1ml of solution b pre-cooled to 4°C for every 100mg of tissue, and cut the tissue into small pieces of 1 mm cube with ophthalmic scissors. Homogenize with a tissue homogenizer. Use Thermo's cocktail mix of protease inhibitors. Thermo Fisher, Protease Inhibitor Cocktail (100X), Cat. No.: 78430.
[0036] 4. Centrifuge the homogenized tissue suspension with a centrifugal force of 700g at 4°C for 10 minutes, take out the supernatant and transfer it to a new centrifuge tube, and discard the precipitate.
[0037] 5. Add 0.1 times the volume of so...
Embodiment 3
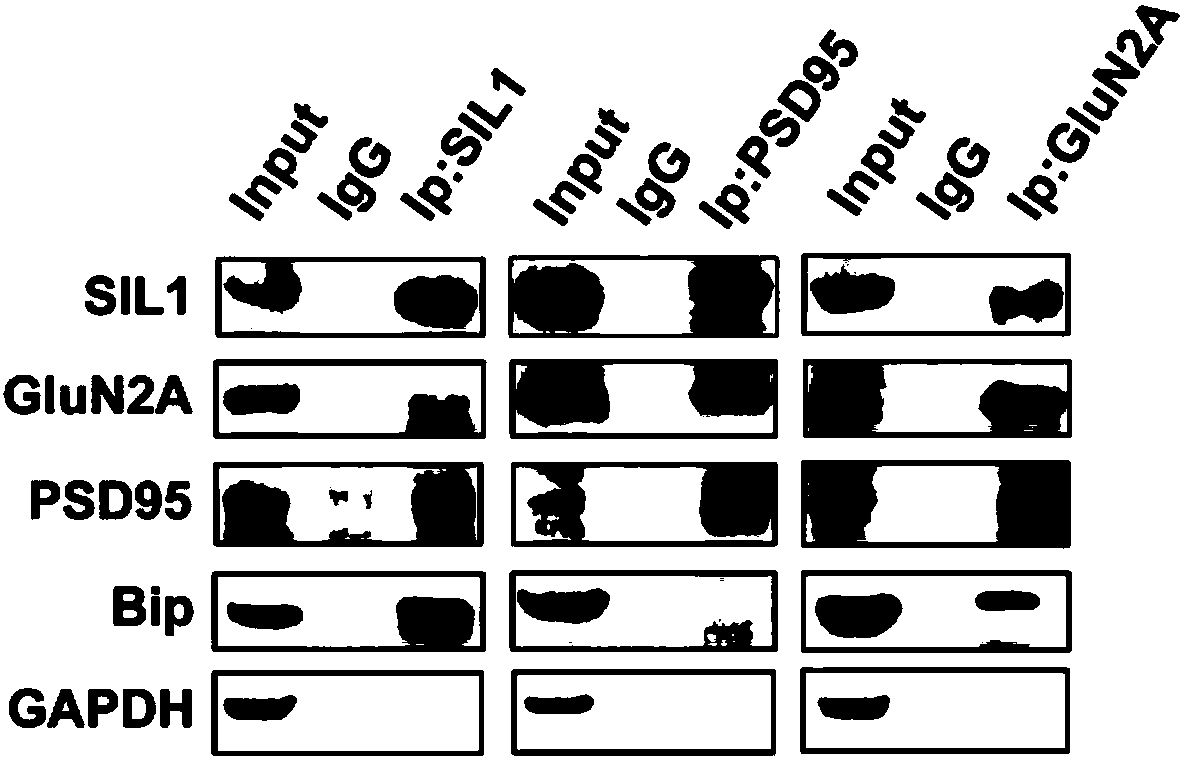
[0046] After using the present invention to extract proteins, the interaction between proteins was analyzed using co-immunoprecipitation method:
[0047] 1. Take mice of different ages, namely mice and female mice born at 1, 4, 7, 10, 13, 16, 19, 22, and 25 days old.
[0048] 2. The mice were killed by neck dislocation, the brain tissue was quickly removed, and the cortex was separated.
[0049] 3. Weigh the isolated cortical tissue, add 1ml of solution b pre-cooled to 4°C for every 100mg of tissue, and cut the tissue into small pieces of 1 mm cube with ophthalmic scissors. Homogenize with a tissue homogenizer. Use Thermo's cocktail mix of protease inhibitors. Thermo Fisher, Protease Inhibitor Cocktail (100X), Cat. No.: 78430.
[0050] 4. Centrifuge the homogenized tissue suspension with a centrifugal force of 700g at 4°C for 10 minutes, take out the supernatant and transfer it to a new centrifuge tube, and discard the precipitate.
[0051] 5. Add 0.1 times the volume of sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com