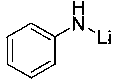
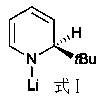
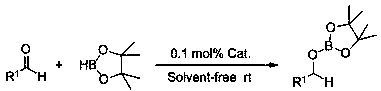Method of preparing borate ester based on aldehyde
A technology for boronate ester and borane, which is applied in the field of preparing boronate ester, can solve the problems of weakening the nucleophilic addition activity of carbonyl, weakening the positive charge of carbonyl carbon, reducing the activity of carbonyl, etc., and achieves simple and controllable reaction, good generalization and the like. adaptability, and the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Lithium anilide catalyzes the hydroboration reaction of cyclohexylbenzaldehyde and pinacol borane
[0026] In the reaction flask that has been dehydrated and deoxygenated, add 20ul tetrahydrofuran solution (0.05M) of lithium anilide (0.1 mol% dosage, the same below) under the protection of argon, then add 0.1596 mL borane with a syringe, mix well, and then use Add 0.095 mL of 2-pyridinecarbaldehyde into the syringe, and stir the mixture at room temperature. After 10 min of reaction, the NMR yield is 99%. After that, a small amount of tetrahydrofuran and excess borane are removed under reduced pressure to obtain the corresponding pinacol borate C 6 h 5 COCH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 8.61 (d, J = 5.4 Hz, 1H, Ar-H), 7.91 (t, J = 7.7 Hz, 1H, Ar-H), 7.49-7.41 (m, 2H, Ar-H), 5.10 (s, 2H, OCH 2 ), 1.32 (s,12H, CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ 159.82 (Ar-C), 143.72 (Ar-C), 139.56(Ar-C), 123.39 (Ar-C), 120.09 (Ar-C...
Embodiment 2
[0027] Example 2: Lithium anilide catalyzes the hydroboration reaction of propionaldehyde and pinacol borane
[0028] In the reaction flask that has been dehydrated and deoxygenated, add 20ul tetrahydrofuran solution (0.05M) of lithium anilide (0.1 mol% dosage, the same below) under the protection of argon, then add 0.1596 mL borane with a syringe, mix well, and then use 0.072 mL of propionaldehyde was added into the syringe, and the mixture was stirred at room temperature. After 10 min of reaction, the NMR yield was 99%. After that, a small amount of tetrahydrofuran and excess borane were removed under reduced pressure to obtain the corresponding pinacol borate CH 3 CH 2 COCH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 3.80 (t, J = 6.6 Hz, 2H, OCH 2 ), 1.63-1.54 (m,2H, CH 2 ), 1.25 (s, 12H, CH 3 ), 0.91 (t, J = 7.4 Hz, 3H, CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ 82.04 (OC), 66.02 (OCH 2 ), 24.14 (CH 3 CH 2 ), 24.05 (CH 3 ), 9.55 (CH 2 CH ...
Embodiment 3
[0031] Example 3: Lithium anilide catalyzes the hydroboration reaction of n-heptanal and pinacol borane
[0032] In the reaction flask that has been dehydrated and deoxygenated, add 20ul tetrahydrofuran solution (0.05M) of lithium anilide (0.1 mol% dosage, the same below) under the protection of argon, then add 0.1596 mL borane with a syringe, mix well, and then use 0.1392 mL of n-heptanal was added into the syringe, and the mixture was stirred at room temperature. After 10 min of reaction, the NMR yield was 99%. After that, a small amount of tetrahydrofuran and excess borane were removed under reduced pressure to obtain the corresponding pinacol borate C 6 h 13 COCH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 3.82 (t, J = 8Hz, 2H, OCH 2 ), 1.52-1.58 (m,2H, CH 2 ), 1.27-1.34 (m, 8H, CH 2 ), 1.24 (s, 12H, CH 3 ), 0.87 (t, J = 8 Hz, 3H, CH 3 ). 13 C NMR (100 MHz, CDCl 3 ) δ 82.02 (OC), 64.40 (OCH 2 ), 31.29 (CH 2 ), 30.92(CH 2 ), 28.44 (CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com