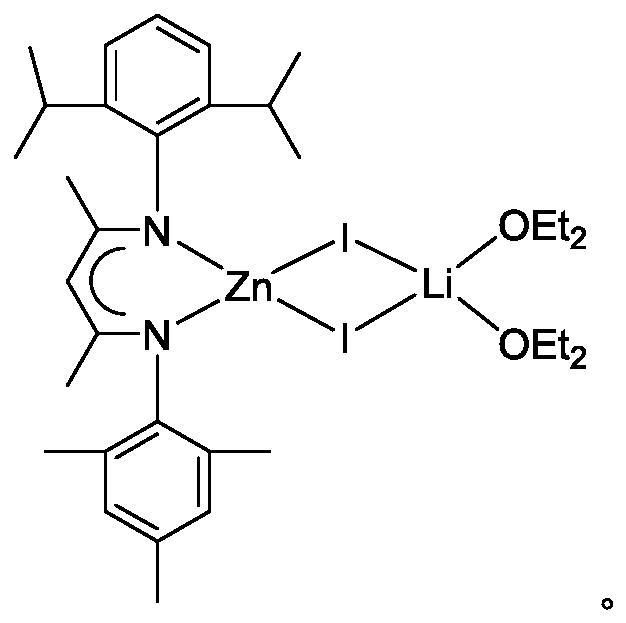
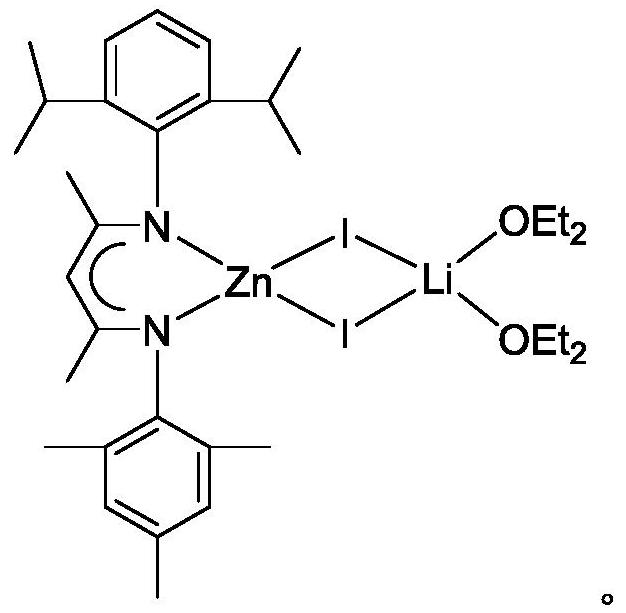
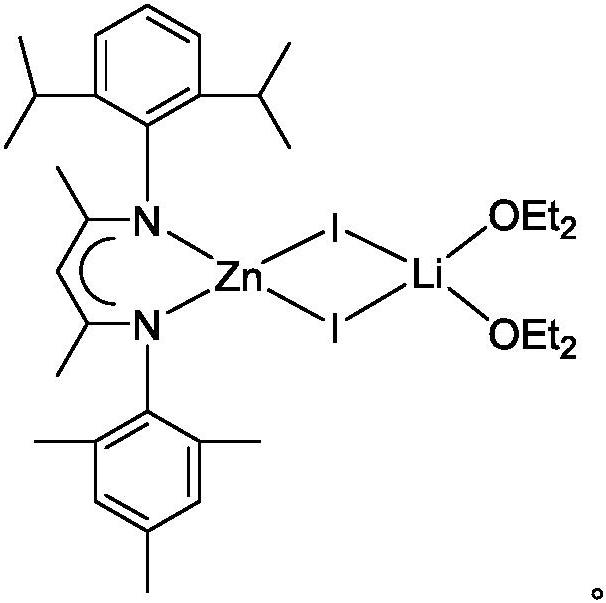
A kind of β-diimine zinc-lithium double metal compound and its preparation method and application in hydroboration of isocyanate
A technology of zinc diimide and isocyanate, which is applied in the field of β-diimide zinc-lithium bimetallic compound and its preparation, and achieves the effects of high activity, high catalytic efficiency and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of β-diimine zinc lithium double metal compound, the process is as follows:
[0024] (1) Under anhydrous and oxygen-free conditions, in a single-port reaction tube, dissolve the β-diimine ligand in ether solution, add n-butyl lithium dropwise to the above solution at -80~-40°C, and return to room temperature for reaction Overnight, obtain β-diimine ligand lithium salt; Wherein, the molar ratio of β-diimine ligand, n-butyllithium is 1: 1.1;
[0025] (2) Add the above solution dropwise to the ether solution of zinc iodide at -80~-40°C, return to room temperature and react for 8~24 hours to obtain β-diimide zinc-lithium bimetallic compound; among them, β-diimide The molar ratio of imine ligand lithium salt and zinc iodide is 1:1. The product was filtered, and the filtrate was concentrated to about 5 mL, and 1.63 g of colorless crystals were precipitated at 2°C, with a yield of 72%. 1 H NMR (600MHz, C 6 D. 6 ): δ7.13(m, 3H, Ar-H), 6.81(m, 2H, Ar-H), 4.95...
Embodiment 2
[0027] β-diimine zinc lithium double metal compound catalyzes the reaction of isopropyl isocyanate and pinacol borane, the process is as follows:
[0028] Under anhydrous and oxygen-free conditions, add 0.29 μmol β-diimide zinc lithium bismetal compound, 5.88 μmol isopropyl isocyanate and 20.58 μmol pinacol borane to a single-port reaction tube in sequence, and use 0.5 mL C 6 D. 6 Dissolve, add a stirring bar and stir at 60° C. for 6 h, and the yield is 99% according to nuclear magnetic spectrum.
Embodiment 3
[0030] The β-diimine zinc-lithium double metal compound catalyzes the reaction of ethyl isocyanate and pinacol borane, and the process is as follows:
[0031] Under anhydrous and oxygen-free conditions, add 0.29 μmol β-diimide zinc-lithium bimetallic compound, 5.88 μmol ethyl isocyanate and 20.58 μmol pinacol borane to a single-port reaction tube in sequence, and use 0.5 mL C 6 D. 6 Dissolve, add a stirring bar and stir at 60° C. for 6 h, and the yield is 99% according to nuclear magnetic spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com