D,D-carboxypeptidase DacA mutant with improved catalysis efficiency and preparation method thereof
A carboxypeptidase and mutant technology, which is applied in the field of D,D-carboxypeptidase DacA mutant and its preparation, can solve the problem of discontinuous protein production, shorten the time of enzymatic property modification and improve the secretion of extracellular protein The effect of improving expression level and catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: D, D-carboxypeptidase DacA catalytic efficiency site-directed mutation analysis and method
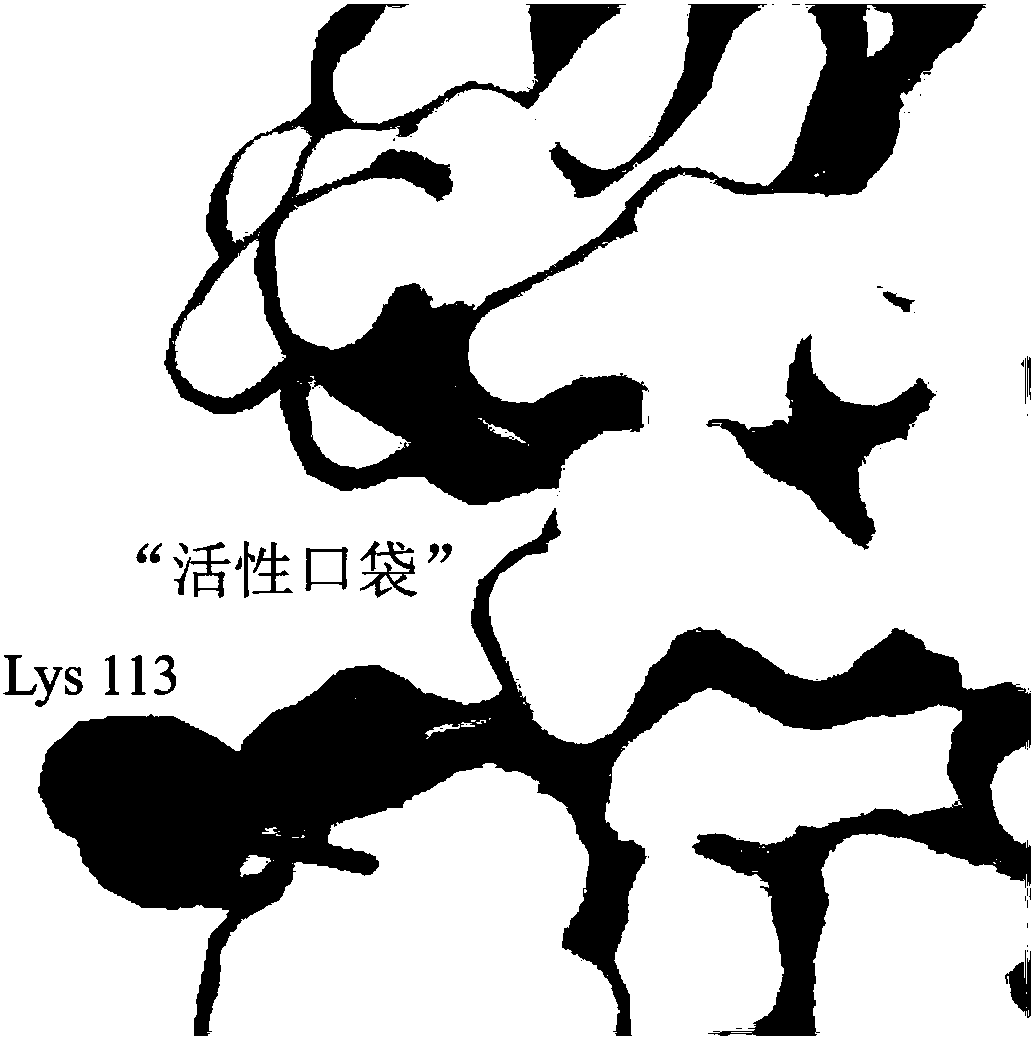
[0016] D,D-carboxypeptidase DacA (SEQ ID NO.1) derived from Escherichia coli was simulated by Swiss-model software to obtain a 3D structure model of D,D-carboxypeptidase DacA. Select the key amino acid residue located in the "active pocket" - lysine, and replace it with arginine, glycine or histidine.
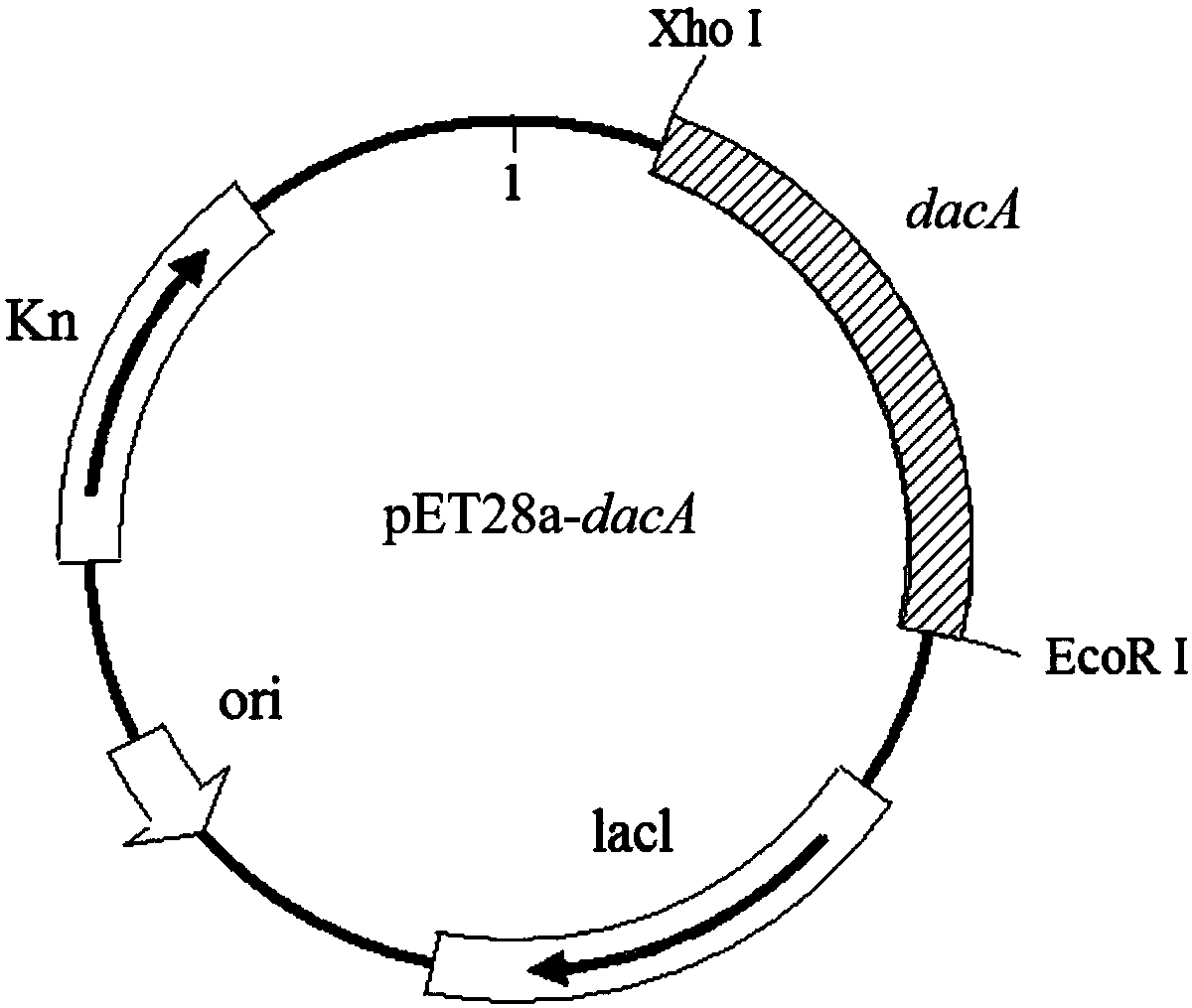
[0017] According to the E.coli D,D-carboxypeptidase DacA sequence, the E.coli BL21 genome was used as a template and cloned between the restriction sites XhoI and EcoRI of the plasmid pET-28a(+) by PCR method to construct the recombinant plasmid pET28a- dacA.
[0018] For site-directed mutagenesis, design corresponding site-directed mutagenesis primers (Table 1). D,D-carboxypeptidase DacA was subjected to site-directed mutation using site-directed mutagenesis primers and recombinant plasmid pET28a-dacA. PCR enzyme was used to amplify the recombinant plasmid pET28a-d...
Embodiment 2
[0021] Embodiment 2: D, D-carboxypeptidase DacA enzyme activity determination and analysis method
[0022] (1) Preparation of D-alanine standard curve: D-alanine solutions with different concentrations of 0-60 mM were prepared. Add 5 μL o-dianisidine solution, 70 μL enzyme and coenzyme (39:19:10:2) mixture, and react at 37°C for 5 minutes. Immediately add 400 μL of methanol / water (1:1) mixture, react at 37°C for 2 min, and measure the absorbance value at 460 nm. Take the concentration of D-alanine as the abscissa and the absorbance as the ordinate to make a standard curve.
[0023] (2) The reaction system includes: 15 μL substrate Park nucleotide analog (Na,Ne-Diacetyl-Lys-D-Ala-D-Ala), 3 μL Tris-HCl buffer (pH 7.5) and 12 μL enzyme solution. Mix well and react at 37°C for 10min. Add 5 μL o-dianisidine, 70 μL enzyme and coenzyme mixture, and react at 37°C for 5 minutes. Immediately add 400 μL of methanol / water mixture, react at 37°C for 2 min, and measure the absorbance va...
Embodiment 3
[0027] Example 3: Determination and analysis of D, D-carboxypeptidase DacA and its mutant catalytic efficiency
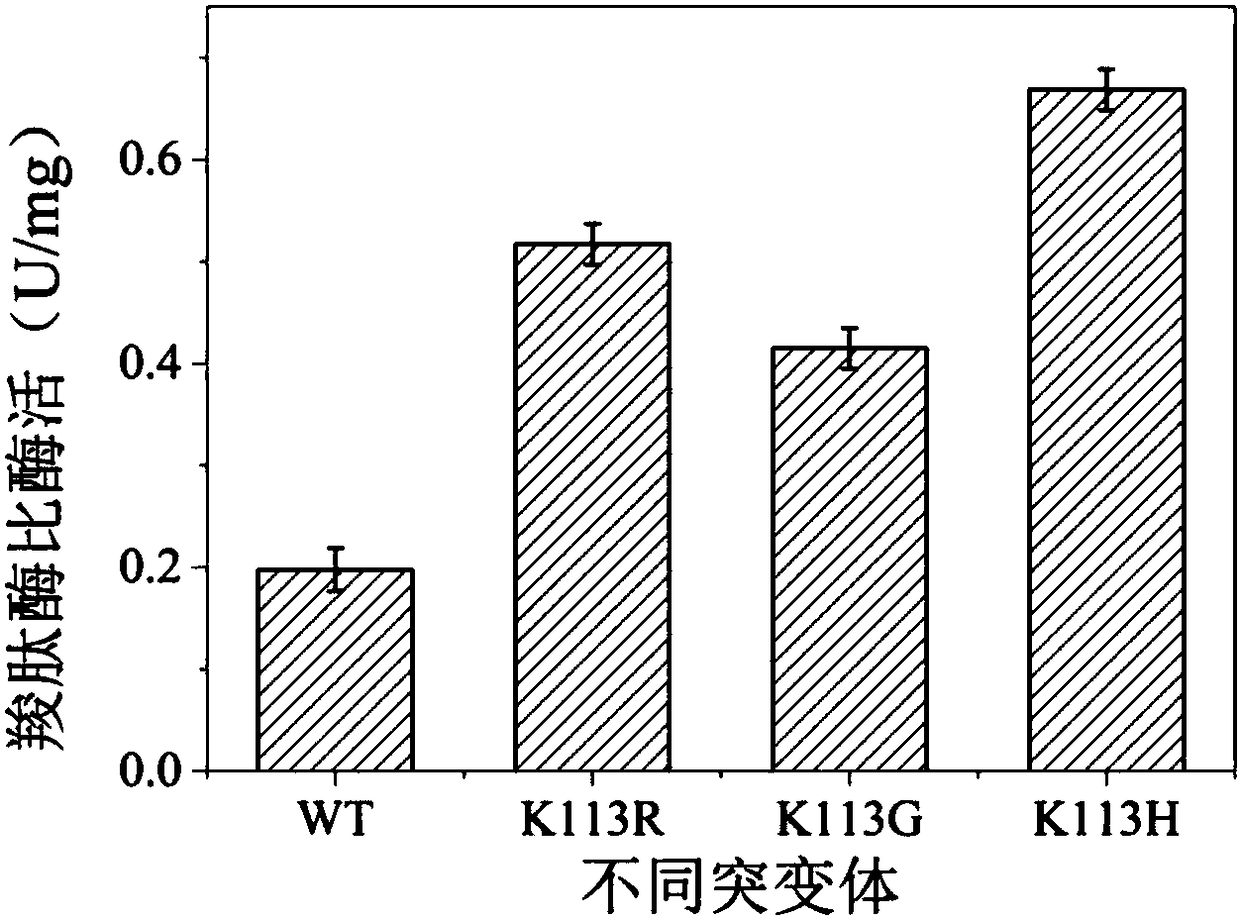
[0028] By measuring the catalytic efficiency of D,D-carboxypeptidase DacA and its mutants, it was found that the catalytic efficiency of the mutants K113R and K113G (Table 2) was increased, but the catalytic efficiency of the mutant K113H was decreased. Catalytic efficiency constant k of mutants K113R and K113G cat values were 6543.42s from wild enzyme -1 Increased to 8541.56s -1 、9428.13s -1 . Among them, the effect of the mutant K113G is the most significant, and the catalytic efficiency constant k cat The value is increased to 1.4 times of the original value. Meanwhile, the K of mutant K113G m The value decreased from 0.85mmol / L of the wild enzyme to 0.66mmol / L, indicating that the binding ability of the enzyme to the substrate was enhanced after the mutation.
[0029] Table 2 Kinetic parameters of D,D-carboxypeptidase DacA mutants
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com