Hypochlorous acid fluorescent probe as well as preparation method and application thereof
A fluorescent probe, hypochlorous acid technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis process of the novel hypochlorous acid fluorescent probe is as follows:
[0033]
[0034] Specific steps are as follows:
[0035] 1) Preparation of 7-(4'-diethylaminobenzylidene) isolongifanone:
[0036] Add 8mmol isolongifolanone, 10mmol p-diethylaminobenzaldehyde, 8mmol sodium ethoxide and 30mL ethanol in sequence to a three-necked flask equipped with a stirrer, thermometer and reflux condenser, and heat to reflux at 80-90°C for reaction , react for about 5 hours until the conversion rate of isolongifolanone reaches over 95% (GC tracking detection). The reactant was extracted 3 times with 30 mL of ethyl acetate, the organic phases were combined, and washed several times with saturated brine until neutral; the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated to recover the solvent to obtain 7-(4′-di The crude product of ethylaminobenzylidene) isolongifolanone is recrystallized with ethanol-ethyl acetate to obtain colorl...
Embodiment 2
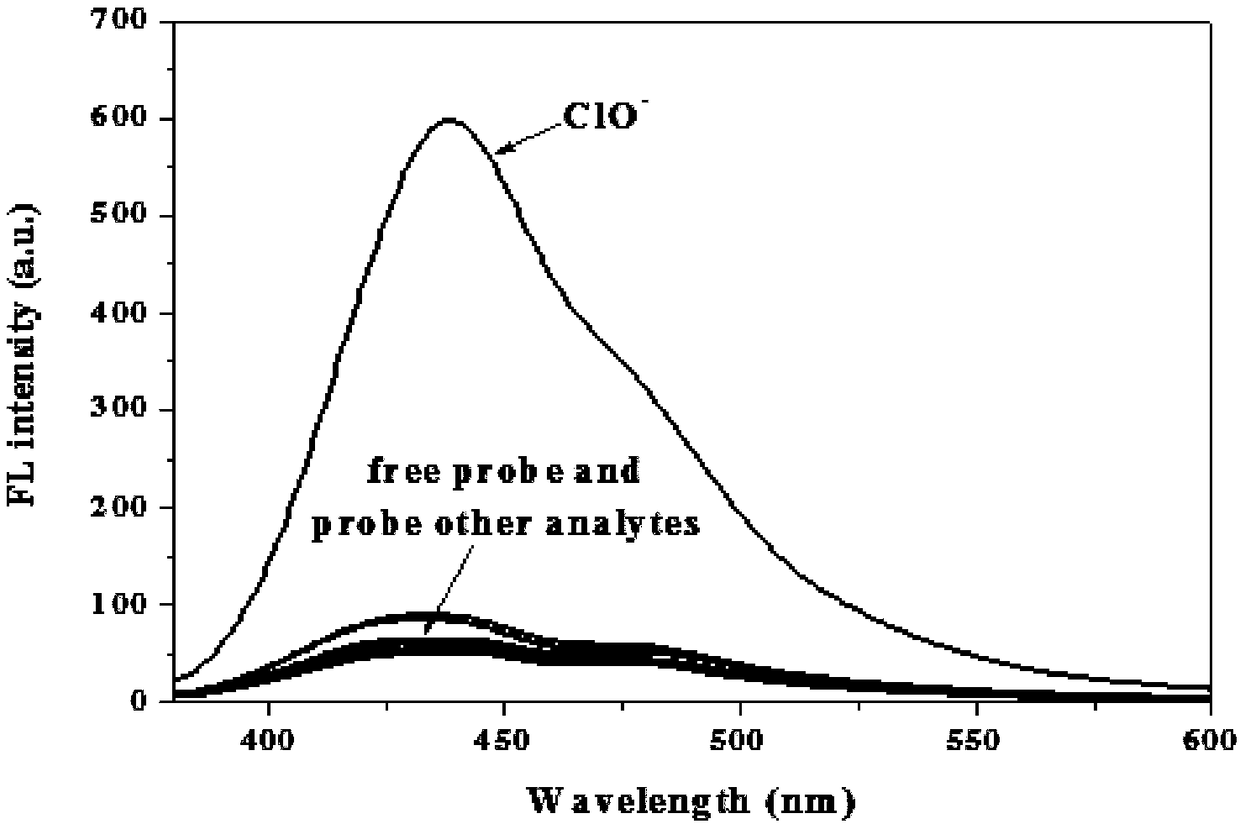
[0048] 1-(((4-(4′-(diethylamino)phenyl)-6,6,10,10-tetramethyl-5,7,8,9,10,10α-hexahydro-6H- 6α,9-Methylenebenzo[h]-2-quinolyl)imino)methyl)naphthalene-2-ol was dissolved in PBS buffer solution (pH=7.4, 10mM, 50% (v / v ) ethanol), prepared into a solution with a concentration of 10 μM, and also active oxygen and anions such as H 2 o 2 、ONOO - , O 2 - , NO 2 - , F - , Cl - 、Br - , NO 3 - 、HSO 3 - , SO 3 2- , SO 4 2- 、HS - 、HCO 3 - , CO 3 2- 、CN - 、SCN - , ClO 2 - , ClO 4 - , ClO -Dissolve in PBS buffer solution to make a solution with a concentration of 1mM. Different reactive oxygen species and anion pairs were measured for 1-(((4-(4′-(diethylamino)phenyl)-6,6,10,10-tetramethyl-5,7,8,9,10 , the fluorescence spectrum of 10α-hexahydro-6H-6α,9-methanobenzo[h]-2-quinolyl)imino)methyl)naphthalene-2-ol, such as figure 1 shown. The results showed that compared with other reactive oxygen species and anions, only hypochlorous acid could cause significant ch...
Embodiment 3
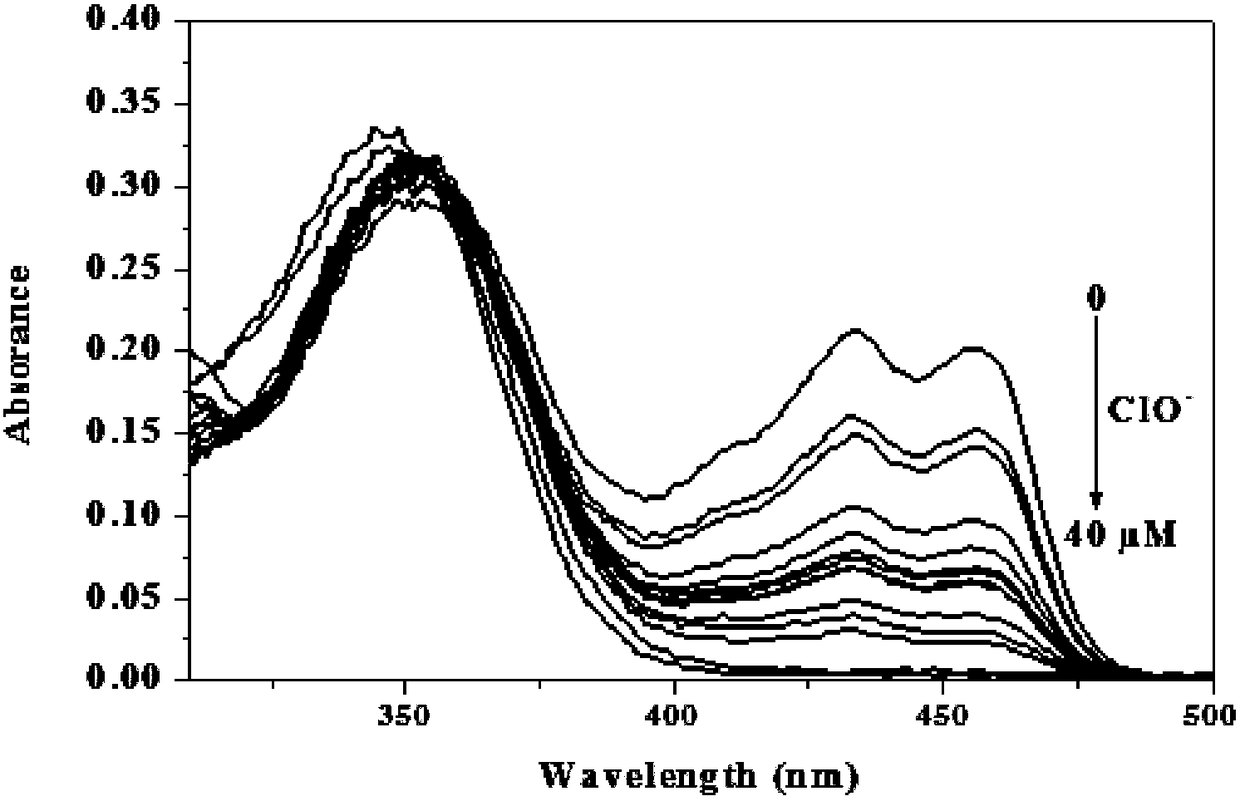
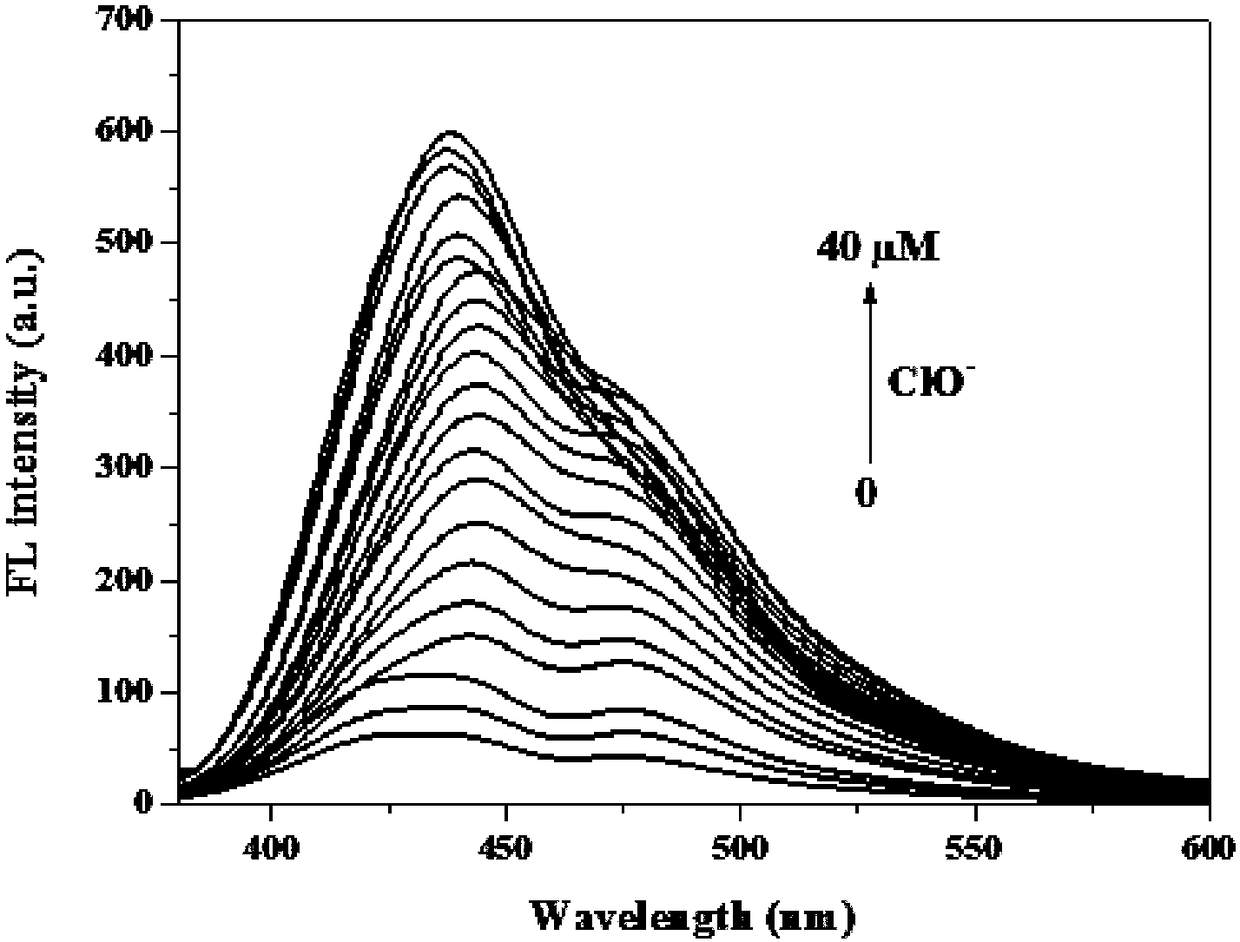
[0050] 1-(((4-(4′-(diethylamino)phenyl)-6,6,10,10-tetramethyl-5,7,8,9,10,10α-hexahydro-6H- 6α,9-Methylenebenzo[h]-2-quinolyl)imino)methyl)naphthalene-2-ol was dissolved in PBS buffer solution (pH=7.4, 10mM, 50% (v / v ) ethanol) to prepare a solution with a concentration of 10 μM, and also dissolve hypochlorous acid in PBS buffer solution to prepare a concentration of 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40 μM solutions. 1-(((4-(4'-(diethylamino)phenyl)-6,6,10,10-tetramethyl-5,7,8,9,10 ,10α-hexahydro-6H-6α,9-methanobenzo[h]-2-quinolyl)imino)methyl)naphthalene-2-ol UV absorption spectrum, such as figure 2 shown. The results showed that the ultraviolet absorption of the compound around 430-450nm decreased significantly, indicating that the compound could react with hypochlorous acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com