Synthesis method for disperse dye intermediate
A technology of disperse dyes and synthesis methods, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc. It can solve the problems of large amount of three wastes, many by-products, and difficult temperature control, etc., and achieves obvious technological advantages. The effect of high conversion rate of hydrogen reduction and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
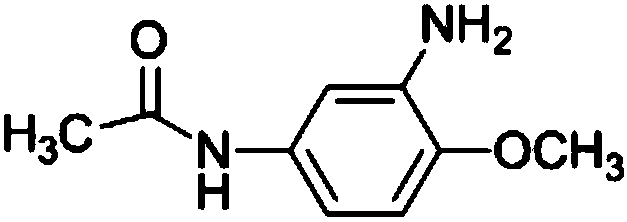
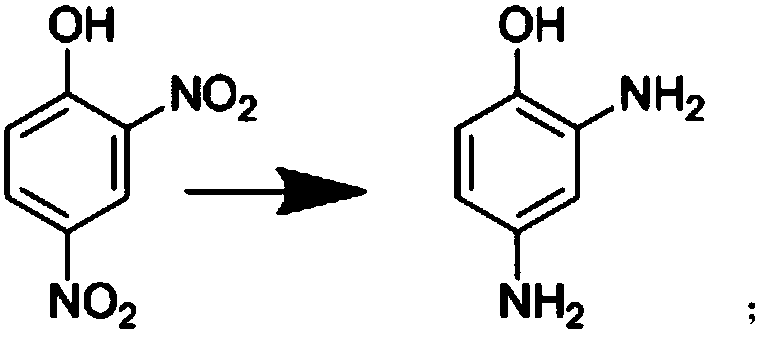
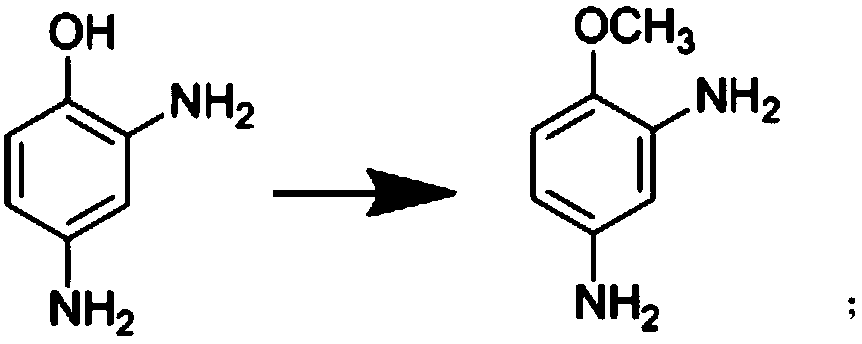
[0032] The synthesis method described in the present invention undergoes three unit reaction processes of hydrogenation reduction, etherification and acylation, using 2,4-dinitrophenol as a raw material, using methanol as a solvent, adding a certain amount of catalyst, and introducing hydrogen to generate reduction Reaction generates 2,4-diaminophenol; The resulting 2,4-diaminophenol and methyl iodide are in the catalyst methyl tributyl methyl ammonium carbonate; Chloromethane is used as a solvent, and the acylation reaction with acetic anhydride generates the target product 3-amino-4-methoxyacetanilide. Concrete reaction steps are as follows:
[0033] 1. Hydrogenation reduction reaction: Put methanol, 2,4-dinitrophenol, and Pd / C catalyst into high pressure in turn, start stirring and record, after stirring for 5 minutes, replace the pressure vessel with nitrogen for 5 times, heat up, and open the hydrogen valve Fill the kettle with hydrogen, the reaction temperature is 60-65...
Embodiment 1
[0039] Step 1,2, the synthesis of 4-diaminophenol
[0040] In a 1000ml autoclave, put 100g of 2,4-dinitrophenol, 202ml of methanol, and 3g of Pd / C catalyst in sequence, stir at room temperature for 5 minutes, replace with nitrogen 5 times, and observe whether the autoclave is leaking, heat up, and open Hydrogen inlet valve, the reaction temperature is 60°C, the pressure in the kettle is kept at 0.35MPa for 2h, adjust the hydrogen inlet valve to make the pressure inside the kettle reach 0.85MPa, keep the temperature for 0.5h, cool down to 40°C, replace with nitrogen 3 times, put The material was suction filtered, the Pd / C catalyst was recovered, and the filtrate was protected with nitrogen for use. Target product 61g, yield 90.52%, purity 98.4%
Embodiment 2
[0042] Step 1,2, the synthesis of 4-diaminophenol
[0043] In a 1000ml autoclave, put 100g of 2,4-dinitrophenol, 316ml of methanol, and 4.5g of Pd / C catalyst in turn, stir at room temperature for 5 minutes, replace with nitrogen 5 times, and observe whether the autoclave is leaking, and then heat up. Open the hydrogen inlet valve, keep the reaction temperature at 62°C, keep the pressure in the kettle at 0.40MPa for 1.5h, adjust the hydrogen inlet valve to make the pressure in the kettle reach 0.85MPa, keep the temperature for 0.5h, cool down to 40°C, and replace with nitrogen 3 times , discharging and suction filtration, recovering the Pd / C catalyst, and the filtrate is protected with nitrogen for use. Target product 63.4g, yield 94.1%, purity 98.7%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com