Method for preparing 6-amino-6-deoxycellulose
A technology of deoxygenated cellulose and cellulose solution, which is applied in the field of natural polymers and their chemical modification, can solve the problems of unfavorable derivatization and application, product insoluble in acidic aqueous solution, removal, etc., and achieve good application prospects and high yield , good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 6-bromo-6-deoxycellulose: adding triphenylphosphine and NBS at 0 °C
[0043] Weigh 4g of dry cellulose into a 500mL three-neck flask, add 220mL of dry DMAc, heat to 130°C, and stir at this temperature for 2 hours. The mixture was cooled to 100° C., 56.5 g of lithium bromide was added, and stirred until the mixture became clear to obtain a cellulose solution. Add 180 mL of dry DMAc and stir at 100°C for 30 minutes. After cooling the cellulose solution with an ice-water bath to 0°C, add a solution of 16.2g of triphenylphosphine dissolved in 40mL of DMAc, stir, and dropwise add a solution of 11.0g of NBS dissolved in 30mL of DMAc, and finish dropping within 30min. The temperature of the reaction liquid was raised to 70° C., and the reaction was stirred for 2 hours. After the reaction was completed, the reaction solution was added dropwise to 1200 mL of distilled water, stirred overnight, filtered, washed with acetone until the filtrate had no UV absorption...
Embodiment 2
[0045] Preparation of 6-bromo-6-deoxycellulose: adding triphenylphosphine and NBS at 5 °C
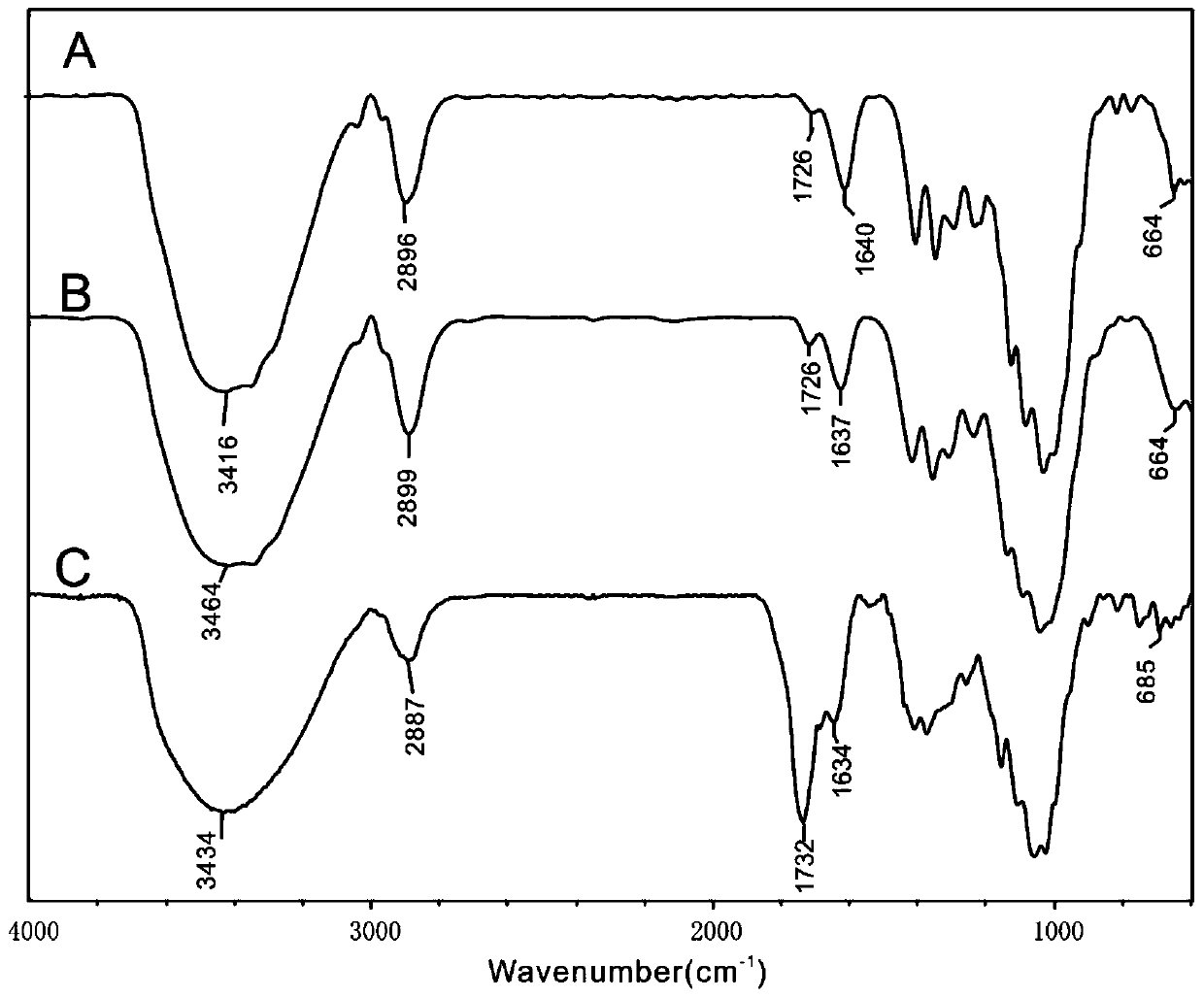
[0046] The temperature of the reaction solution was adjusted to 5°C, and triphenylphosphine and NBS were added at this temperature, and the rest of the method was the same as in Example 1 to prepare 6-bromo-6-deoxycellulose. The productive rate is 97%, and the infrared spectrogram is as attached figure 1 As shown in B, IR(KBr,cm -1 )υ: 3464(-OH), 2899(-C-H), 1726(-O-C=O), 664(-C-Br). in the attached figure 1 1726cm on B -1 Nearby ester group peak and 1637cm -1 Compared with the cellulose skeleton peak of A, it is weaker, and there is no obvious difference with the peak intensity in A spectrum.
Embodiment 3
[0048] Preparation of 6-azido-6-deoxycellulose: The ratio of reaction solvent DMSO to 6-bromo-6-deoxycellulose is 40mL / g
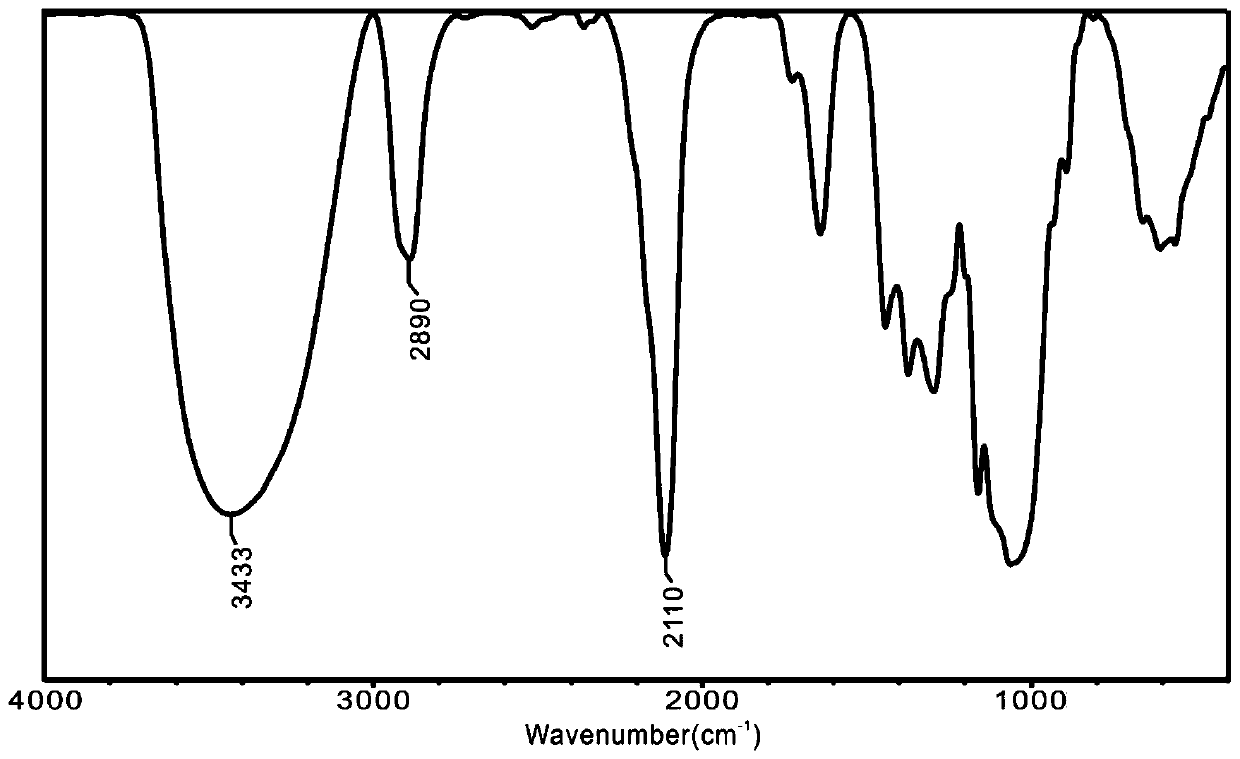
[0049] The ratio of the reaction solvent DMSO and 6-bromo-6-deoxycellulose was adjusted to 40mL / g, and the rest of the method was the same as that in comparative experiment 1 to prepare 6-azido-6-deoxycellulose. The productive rate is 78%, and the infrared spectrogram is as attached figure 2 Shown: IR(KBr,cm -1 )υ: 3433(-OH), 2890(-C-H), 2110(-N3 ), at 2110cm -1 There is a strong azide absorption peak, indicating that 6-azido-6-deoxycellulose is generated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com