Dithioacetal derivative containing methoxyacrylate, preparation method and application thereof
A technology of methoxyacrylate and methyl methoxyiminoacetate, which is applied to dithioacetal derivatives containing methoxyacrylate, their preparation and application fields, and can solve problems such as lack of research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
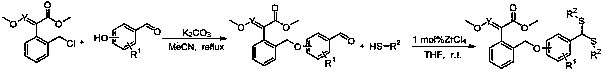
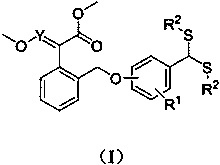
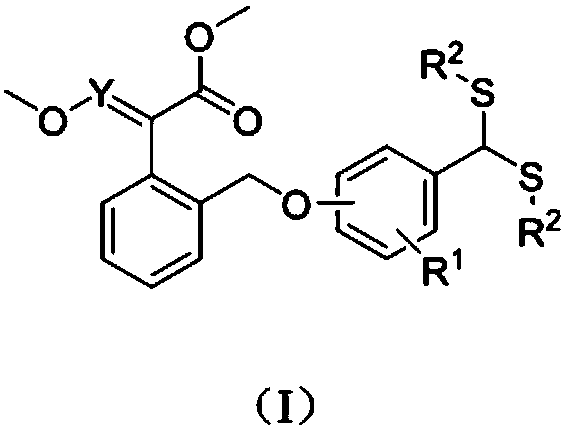
[0044] (E)-2-(((2-methoxy-4-bis(2-hydroxyethyl)dithioacetal)-2-phenoxymethylene)phenyl)-2-methoxymethylene The synthesis of methyl aminoacetate (compound number is 1), comprises the following steps:
[0045] (1) Synthesis of (E)-2-(((2-methoxy-4-formyl)-2-phenoxymethylene)phenyl)-2-methoxyiminoacetic acid methyl ester:
[0046] Add (E)-2-((2-chloromethyl)phenyl)-2-methoxyiminoacetic acid methyl ester (2g, 8.28mmol) and vanillin (1.26g, 8.28mmol) into a 100mL three-necked flask and add 40mL acetonitrile to dissolve the solid, add anhydrous K 2 CO 3 (3.43g, 24.83mmol), the reaction system is yellow turbidity (K 2 CO 3 undissolved), reflux stirring, thin layer chromatography (TLC) tracking reaction process (wherein developing agent is sherwood oil: ethyl acetate=3:1, V / V), after raw material point disappears, stops reaction, spins to dry solvent, Repeatedly washed with water twice, extracted with ethyl acetate, collected the organic phase and spin-dried the solvent to obtain...
Embodiment 2
[0050] (E)-2-(((2-methoxy-4-bis(ethylthio)dithioacetal)-2-phenoxymethylene)phenyl)-2-methoxyiminoacetic acid The synthesis of methyl ester (compound number is 2), comprises the following steps:
[0051] (1) Synthesis of (E)-2-(((2-methoxy-4-formyl)-2-phenoxymethylene)phenyl)-2-methoxyiminoacetic acid methyl ester:
[0052] Synthesize as embodiment 1 (1) method and condition;
[0053] (2) (E)-2-(((2-methoxy-4-di(ethylthio)dithioacetal)-2-phenoxymethylene)phenyl)-2-methoxy Synthesis of methyl iminoacetate:
[0054] Synthesize as embodiment 1 (2) method and condition, difference is that ethanethiol is raw material;
Embodiment 3
[0056] (E)-2-(((2-methoxy-4-bis(4-fluorophenyl)dithioacetal)-2-phenoxymethylene)phenyl)-2-methoxymethylene The synthesis of methyl aminoacetate (compound number is 3), comprises the following steps:
[0057] (1) Synthesis of (E)-2-(((2-methoxy-4-formyl)-2-phenoxymethylene)phenyl)-2-methoxyiminoacetic acid methyl ester:
[0058] Synthesize as embodiment 1 (1) method and condition;
[0059] (2) (E)-2-(((2-methoxy-4-bis(4-fluorophenyl)dithioacetal)-2-phenoxymethylene)phenyl)-2-methyl Synthesis of Methyl Oxyiminoacetate
[0060] Synthesize as embodiment 1 (2) method and condition, difference is that p-fluorothiophenol is raw material;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com