Thermo-sensitive polymer as well as synthetic method and application thereof
A temperature-sensitive polymer and synthesis method technology, applied in the direction of medical preparations and pharmaceutical formulas of non-active ingredients, can solve the problems of narrow adjustable range, unfavorable precise adjustment, etc., and achieve the effect of easy promotion and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
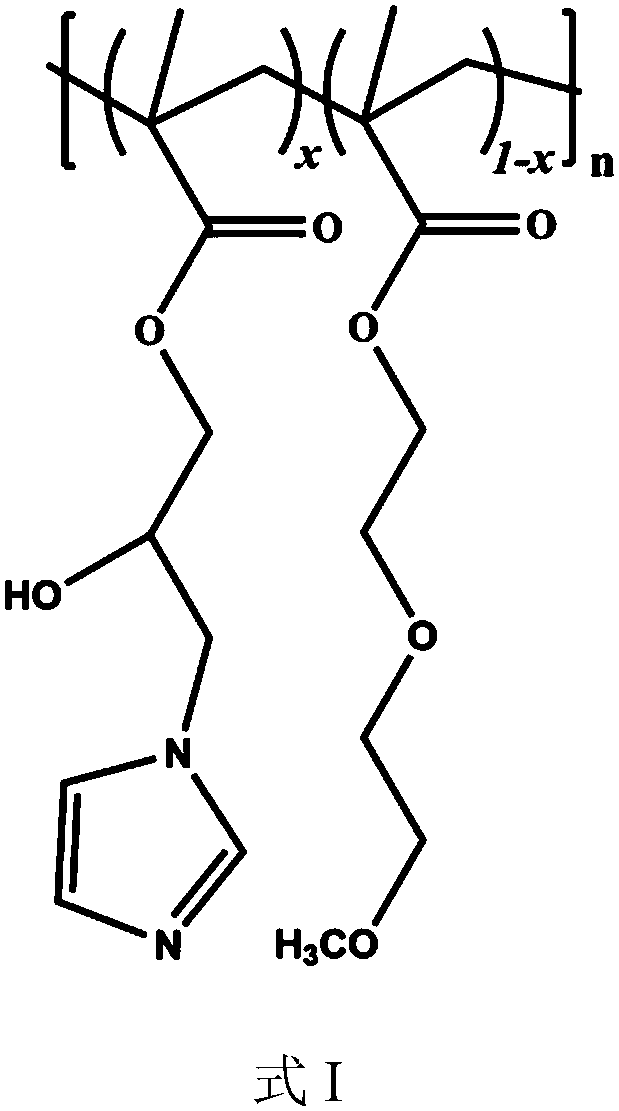
[0037] A thermosensitive polymer prepared by the following method:
[0038] (1) Add 0.71g glycidyl methacrylate, 3.76g 2-(2-methoxyethoxy) ethyl methacrylate, 0.051g trimethylsilylpropyne to a 100ml round bottom flask Ethyl-2-bromoisobutyrate, 0.150g 4,4'-dinonylbipyridyl and 10ml anisole, the reaction system was stirred and dissolved, and argon was bubbled for 30 minutes to remove oxygen, and then 0.026g bromine was added Cuprous chloride was polymerized at 80°C for 2.5 hours. The reaction product was diluted with tetrahydrofuran and passed through a neutral alumina column. The filtrate was concentrated and then precipitated in ether. The product was precipitated and washed twice, and then dried in vacuum at room temperature for 24 hours. To constant weight, obtain 3.87g glycidyl methacrylate and 2-(2-methoxyethoxy) ethyl methacrylate copolymer, product yield is 86.5%.
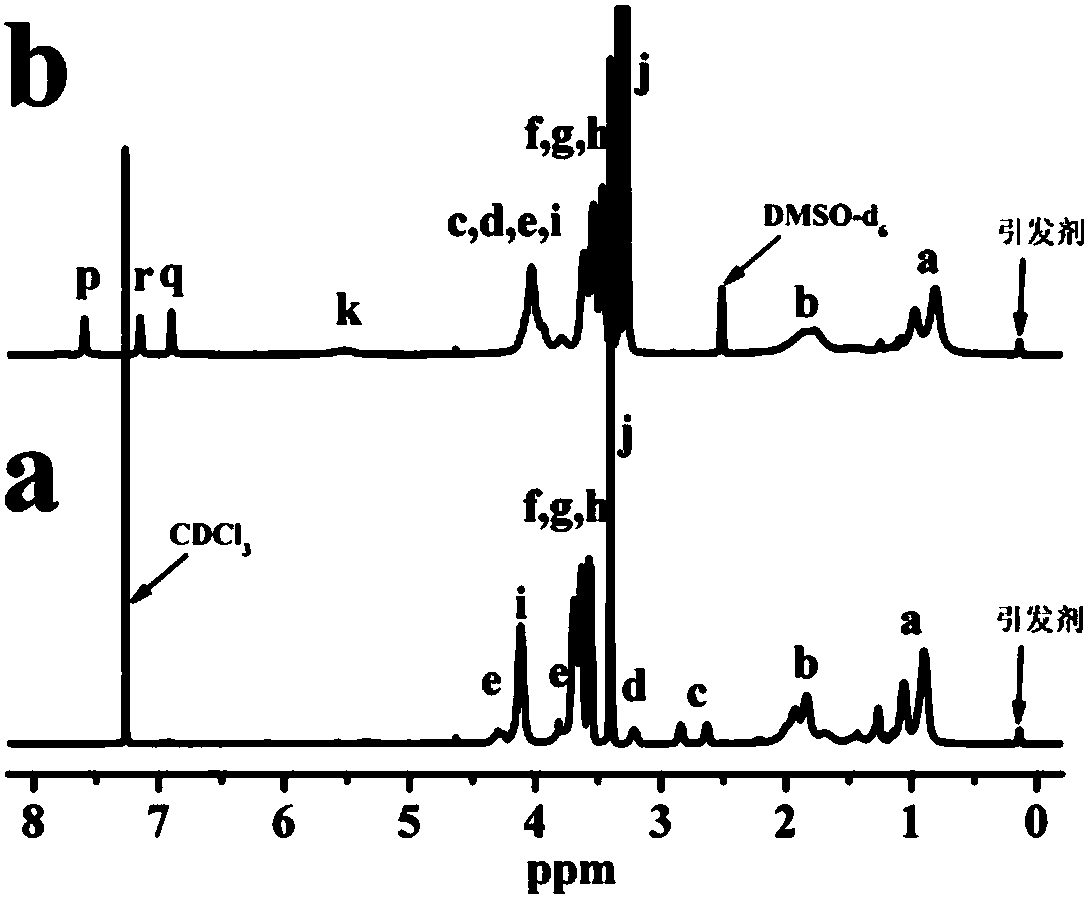
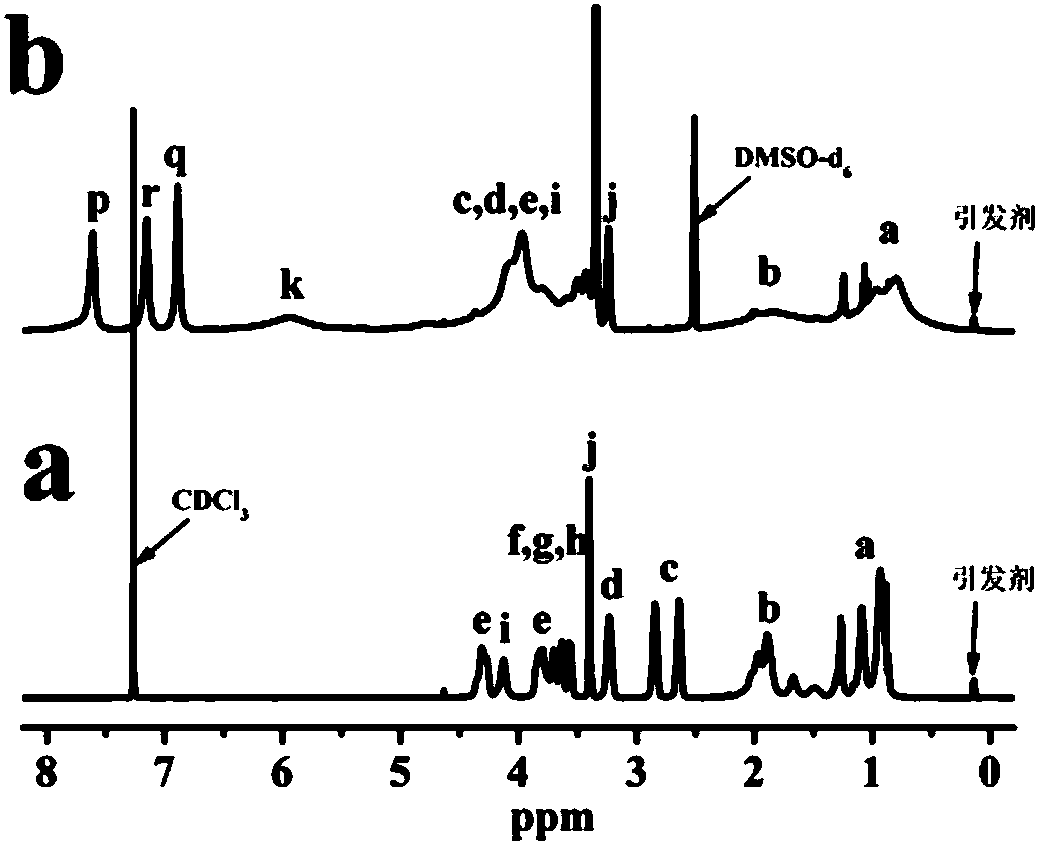
[0039] product 1 H NMR results as figure 1 As shown in (a), the result analysis shows that the degree o...
Embodiment 2
[0044] A thermosensitive polymer prepared by the following method:
[0045] (1) Add 2.84g glycidyl methacrylate, 0.94g 2-(2-methoxyethoxy) ethyl methacrylate, 0.053g trimethylsilylpropyne to a 100ml round bottom flask Ethyl-2-bromoisobutyrate, 0.152g 4,4'-dinonylbipyridyl and 10ml anisole, the reaction system was stirred and dissolved, and argon was bubbled for 30 minutes to remove oxygen, and then 0.025g bromine was added Cuprous chloride was polymerized at 80°C for 1.0 h. The reaction product was diluted with tetrahydrofuran and passed through a neutral alumina column. The filtrate was concentrated and then precipitated in ether. The product was precipitated and washed twice, and then dried in vacuum at room temperature for 24 h. To a constant weight, 3.49 g of glycidyl methacrylate and 2-(2-methoxyethoxy) ethyl methacrylate copolymer were obtained, and the product yield was 90.3%.
[0046] product 1 H NMR results as figure 2 As shown in (a), the result analysis shows th...
Embodiment 3
[0051] A thermosensitive polymer prepared by the following method:
[0052] (1) Add 2.12g glycidyl methacrylate, 2.83g 2-(2-methoxyethoxy) ethyl methacrylate, 0.061g trimethylsilyl propyne to a 50ml round bottom flask Ethyl-2-bromoisobutyrate, 0.183g 4,4'-dinonylbipyridine and 10ml anisole, the reaction system was stirred and dissolved, and argon was bubbled for 30 minutes to remove oxygen, and then 0.032g bromine was added Cuprous chloride was polymerized at 80°C for 1.5 hours. The reaction product was diluted with tetrahydrofuran and then passed through a neutral alumina column. The filtrate was concentrated and then precipitated in ether. The product was precipitated and washed twice, and then dried in vacuum at room temperature for 24 hours. To a constant weight, 4.36 g of glycidyl methacrylate and 2-(2-methoxyethoxy) ethyl methacrylate copolymer were obtained, and the product yield was 88.1%.
[0053] product 1 Analysis of H NMR results showed that the degree of polymer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com