Method for quantitatively analyzing photocyanine isomers by liquid chromatography-ion mobility differential mass spectrometry coupling technology
A technology of isomers and ion mobility, applied in the fields of biochemistry and pharmaceutical analytical chemistry, can solve the problems of unsatisfactory pharmacokinetics, poor analysis sensitivity, long analysis time, etc., and achieve high practicability and reliability , high sensitivity and strong data reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Quantitative analysis of four isomers of fudaine
[0038] This example illustrates the separation and quantification of four isomers of forsaine by high performance liquid chromatography-ion mobility differential mass spectrometry.
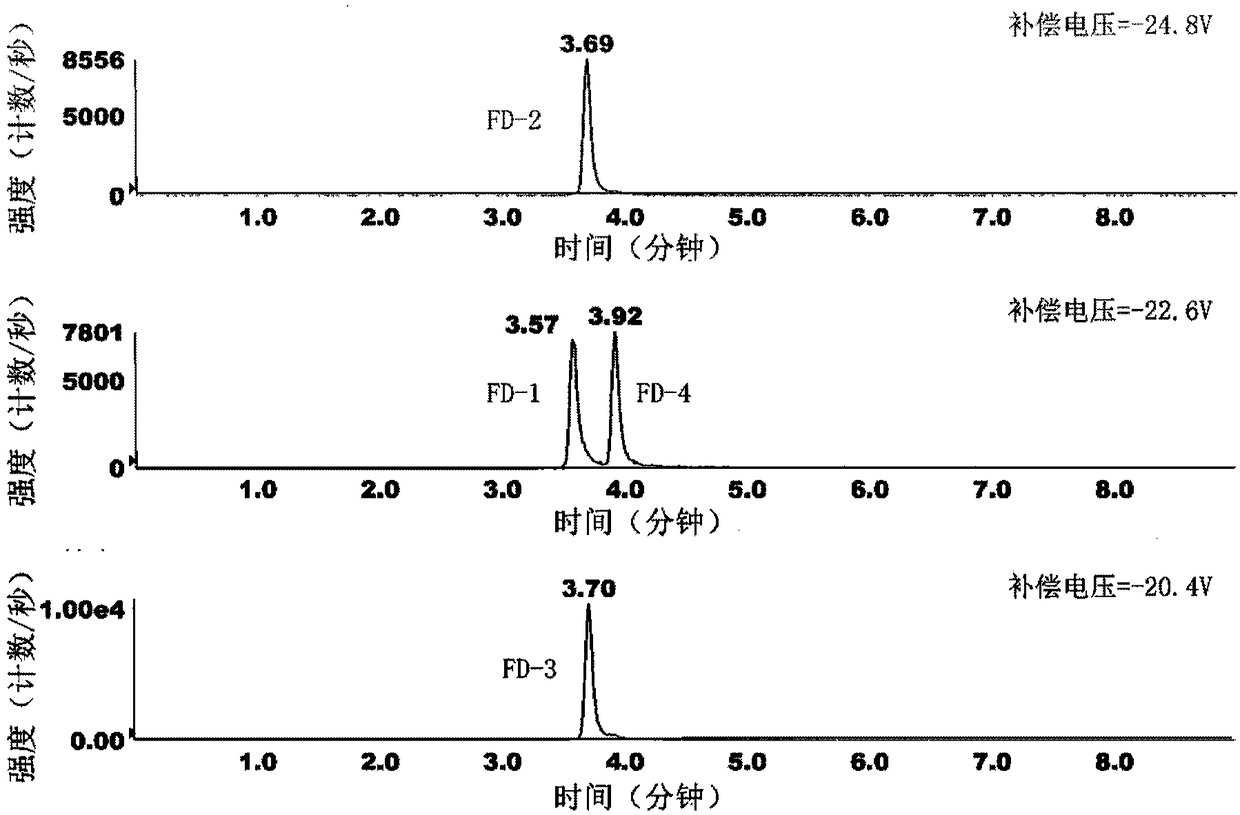
[0039] Solution preparation: Weigh 10 mg of Forsaine mixture powder, dissolve in 10 mL of N, N-dimethylformamide, prepare a solution with a concentration of 1 mg / mL, and use a diluent (methanol: acetonitrile: water = 25:25:50) Diluted to a solution of 800ng / mL, take 10-20 μL of the above solution and inject it into a high-performance liquid chromatography-ion mobility differential mass spectrometry tandem device to obtain a mass chromatogram ( figure 1 ). The four isomers of forsaine are completely separated by chromatography and ion differential two-dimensional separation, so they can be quantified simultaneously.
[0040] High performance liquid chromatography conditions:
[0041] Column: Waters UPLC BEH C 18 Column, spe...
Embodiment 2
[0052] Example 2: Methodological Validation
[0053] This example illustrates the methodological validation of the method for the quantitative analysis of the four isomers of Dacein in plasma.
[0054] Plasma sample preparation method:
[0055] Take 50 μL of plasma containing forsaine, add 200 μL of precipitant (methanol: acetonitrile = 50:50), centrifuge for 5 minutes, take 100 μL of supernatant, add 100 μL of water, mix, take 10-20 μL for injection.
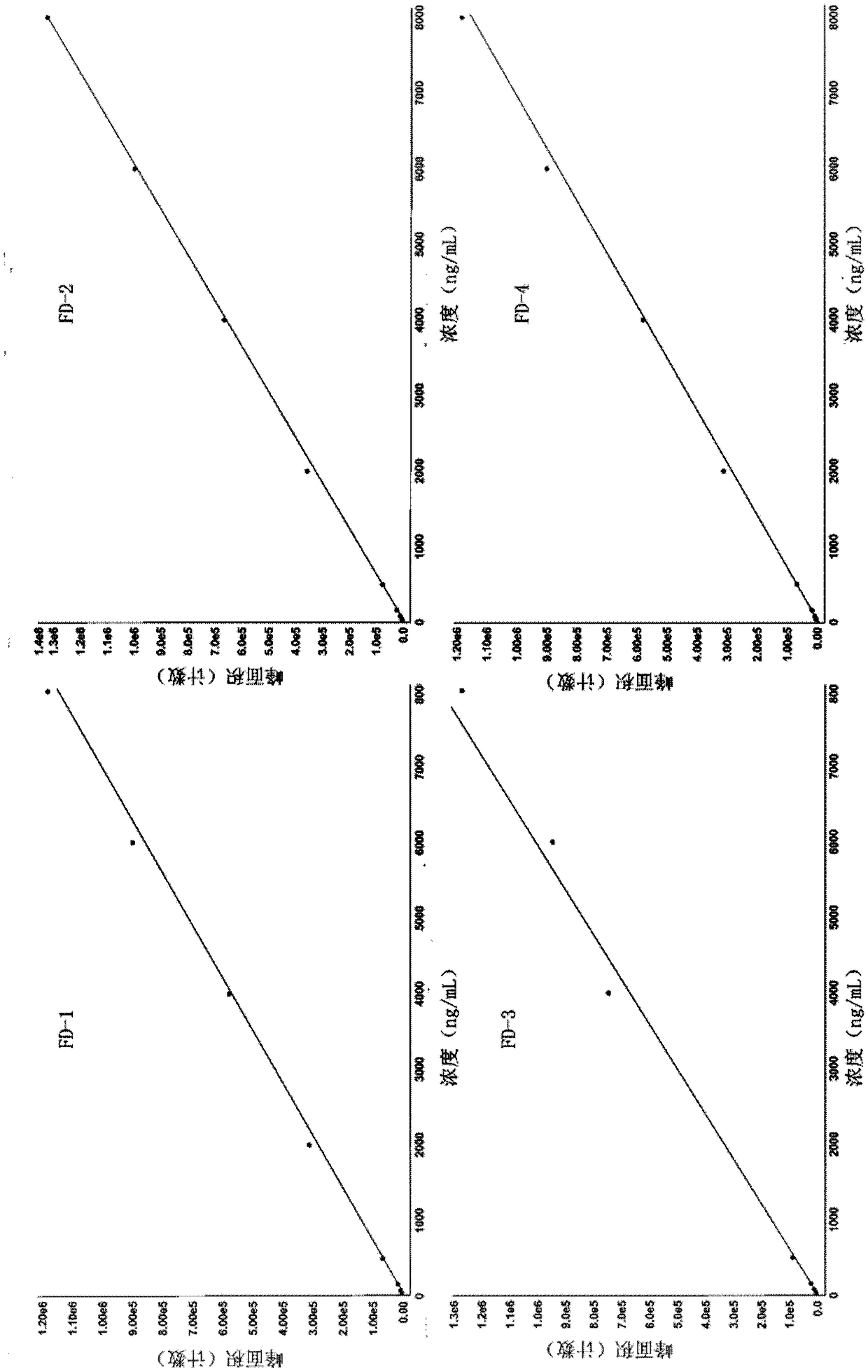
[0056] Adopt the analysis method provided by the invention, use external standard method to carry out quantitative analysis. The method was validated for specificity, standard curve, lower limit of quantitation, precision and accuracy, matrix effects, and recovery.
[0057] A. Specificity: Individual plasma samples from 6 different volunteers were used to measure using the analysis method provided by the present invention. In the obtained mass chromatogram, it can be judged that there is no interference of endogenous substanc...
Embodiment 3
[0068] Embodiment 3: pharmacokinetic research
[0069] This example illustrates the study on the pharmacokinetics of four isomers after intravenous injection of forsaine injection in tumor patients.
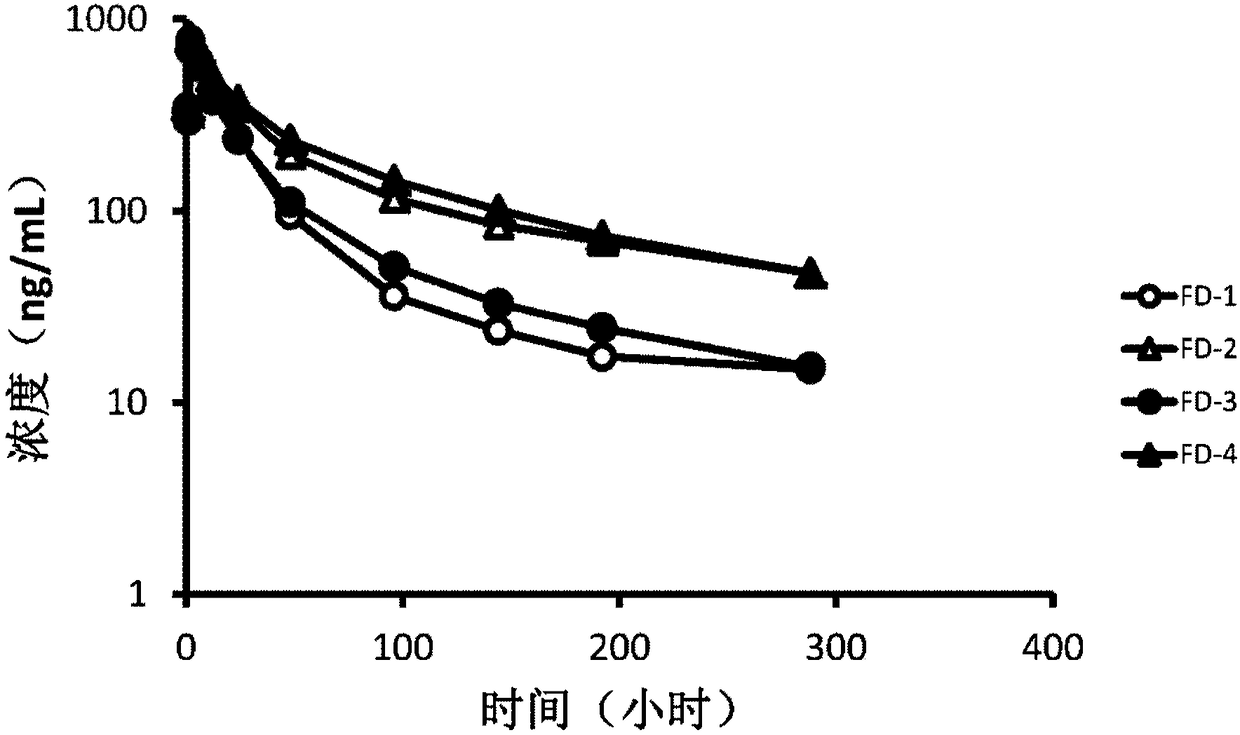
[0070] Patients with advanced esophageal cancer were intravenously injected with 7 mg of forzaine for 1 hour, and 24 hours after administration, they were irradiated with 670nm laser. , 48h, 96h, 144h, 192h and 288h), blood samples (3 mL) were collected, which were centrifuged at 3000 g for 10 minutes at 4° C. to obtain plasma. Using the plasma pretreatment method provided by the present invention and the quantitative analysis method using high performance liquid chromatography-ion mobility differential mass spectrometry tandem device to analyze the pharmacokinetic characteristics of the four isomers of Forzaine in human plasma, to Study the clinical pharmacology of this innovative drug. The results of pharmacokinetic studies in image 3 shown in . Depend on image 3 As can ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com