Lithium-ion battery cathode material li(ni 0.8 co 0.1 mn 0.1 ) 1-x the y x o 2 and preparation method
A lithium-ion battery, 1-xyxo2 technology, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve problems such as poor safety performance and poor electrochemical cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
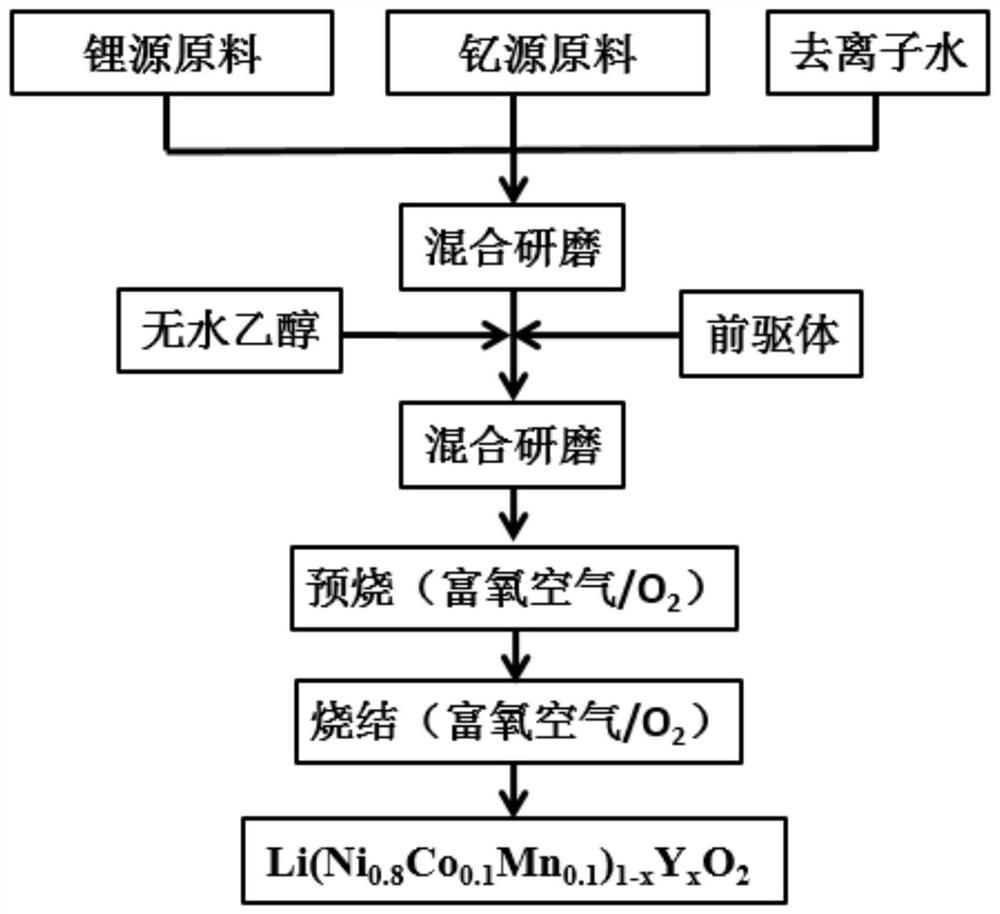
[0031]When the amount of lithium is 10%, 0.02 (ie x = 0.02), respectively, according to the yttrium, and 1.1656 g LiOH · h2O, dissolve in 10 ml of deionized water, add 0.0565G Y2O3Uniform grinding, then add 2.2852 g of precursor and absolute ethanol, ground until the flow-norm mixed slurry is obtained, and then continue to ground under infrared lamp to completely become a mixed powder; then put it in an oven and ground into fine powder. Finally, the fine powder was placed in a tubular furnace under an oxygen atmosphere (oxygen flow rate 400 ml / min) at a rate of 3 ° C / min to warm up to 470 ° C, and then warmed to 780 ° C in a speed of 2 ° C / min. 15h,, then cool down to 450 ~ 500 ° C in 2 ° C / min, finally graining the product after cooling to room temperature, i.e., LI (Ni0.8CO0.1Mn0.1)0.98Y0.02O2.
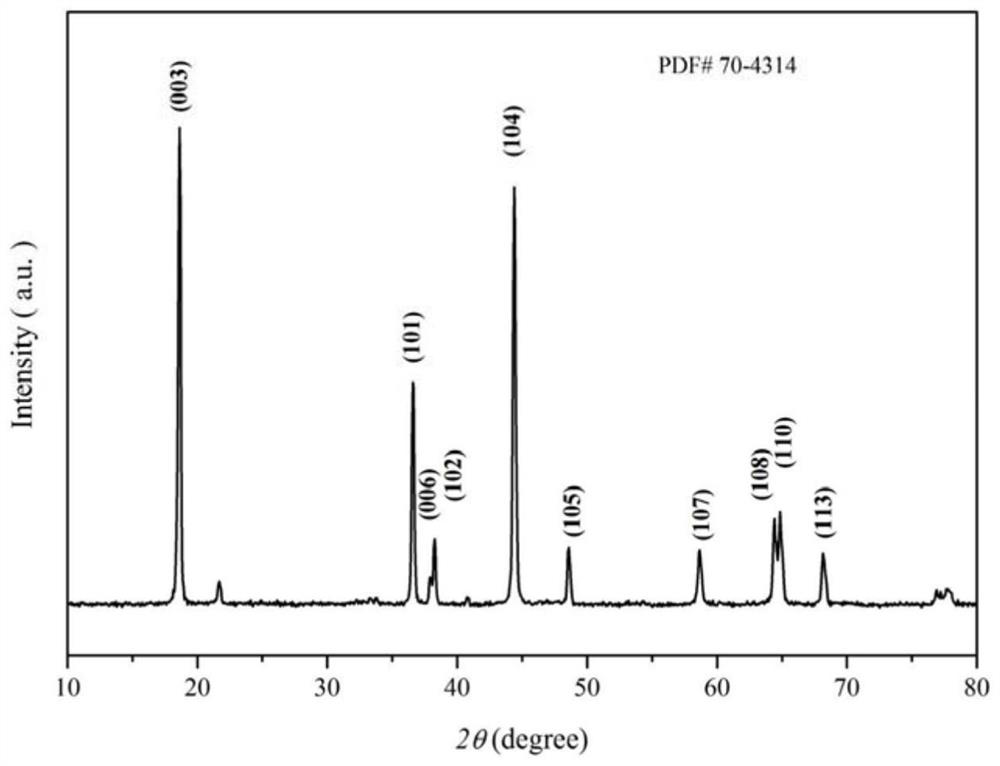
[0032]Preparative lithium ion battery positive material Li (Ni0.8CO0.1Mn0.1)0.98Y0.02O2Perform constant current charge and discharge test, the test results areFigure 2 ~ 7As shown, f...
Embodiment 2
[0035]When the amount of lithium is 5%, 0.02 (i.e., x = 0.02), respectively, 1.1126 g LiOH · h2O, dissolve in 10 ml of deionized water, add 0.0565G Y2O3Uniform grinding, then add 2.2852 g of precursor and anhydrous ethanol, grind until the flow-norm mixed slurry is obtained, and then continuously grinding under the infrared lamp to completely become a mixed powder; then put it in the oven and grind, finally It was raised to the oxygen atmosphere (oxygen flow rate 400 ml / min) at a rate of 3 ° C / min at a speed of 470 ° C for 6 h, and then heated to 780 ° C for 15 h at a rate of 2 ° C / min. Then the 2 ° C / min program is lowered to 450 ~ 500 ° C, and finally the product is ground after cooling to room temperature, i.e., Li (Ni)0.8CO0.1Mn0.1)0.98Y0.02O2.
[0036]Preparative lithium ion battery positive material Li (Ni0.8CO0.1Mn0.1)0.98Y0.02O2Perform constant current charge and discharge test, from the test results, it can be seen that the positive electrode material still has high di...
Embodiment 3
[0039]When the amount of lithium is 10%, 0.01 (i.e., x = 0.01), respectively, according to the 1.1656 g of LiOH · h2O, dissolve in 10ml deionized water, add 0.0283G Y2O3Even with a grinding, 2.2509 g of precursor and anhydrous ethanol were added, grinded until the flow-norm mixed slurry was obtained, and then polished under infrared lamp to completely become a mixed powder; then put it in the oven and grinded, and finally It was raised to the oxygen atmosphere (oxygen flow rate 400 ml / min) at a rate of 3 ° C / min at a speed of 470 ° C for 6 h, and then heated to 780 ° C for 15 h at a rate of 2 ° C / min. Then the 2 ° C / min program is lowered to 450 ~ 500 ° C, and finally the product is ground after cooling to room temperature, i.e., Li (Ni)0.8CO0.1Mn0.1)0.99Y0.01O2.
[0040]Preparative lithium ion battery positive material Li (Ni0.8CO0.1Mn0.1)0.99Y0.01O2Perform constant current charge and discharge test, from the test results, it can be seen that the positive electrode material st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com