Application of cycloastragenol to preparation of drug for inhibiting abdominal aortic aneurysm
A technology for abdominal aortic aneurysm and abdominal aorta, which is applied in the application field of cycloastragenol in the preparation of drugs for inhibiting abdominal aortic aneurysm, achieving the effect of high yield, simple process and strong pharmacological effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
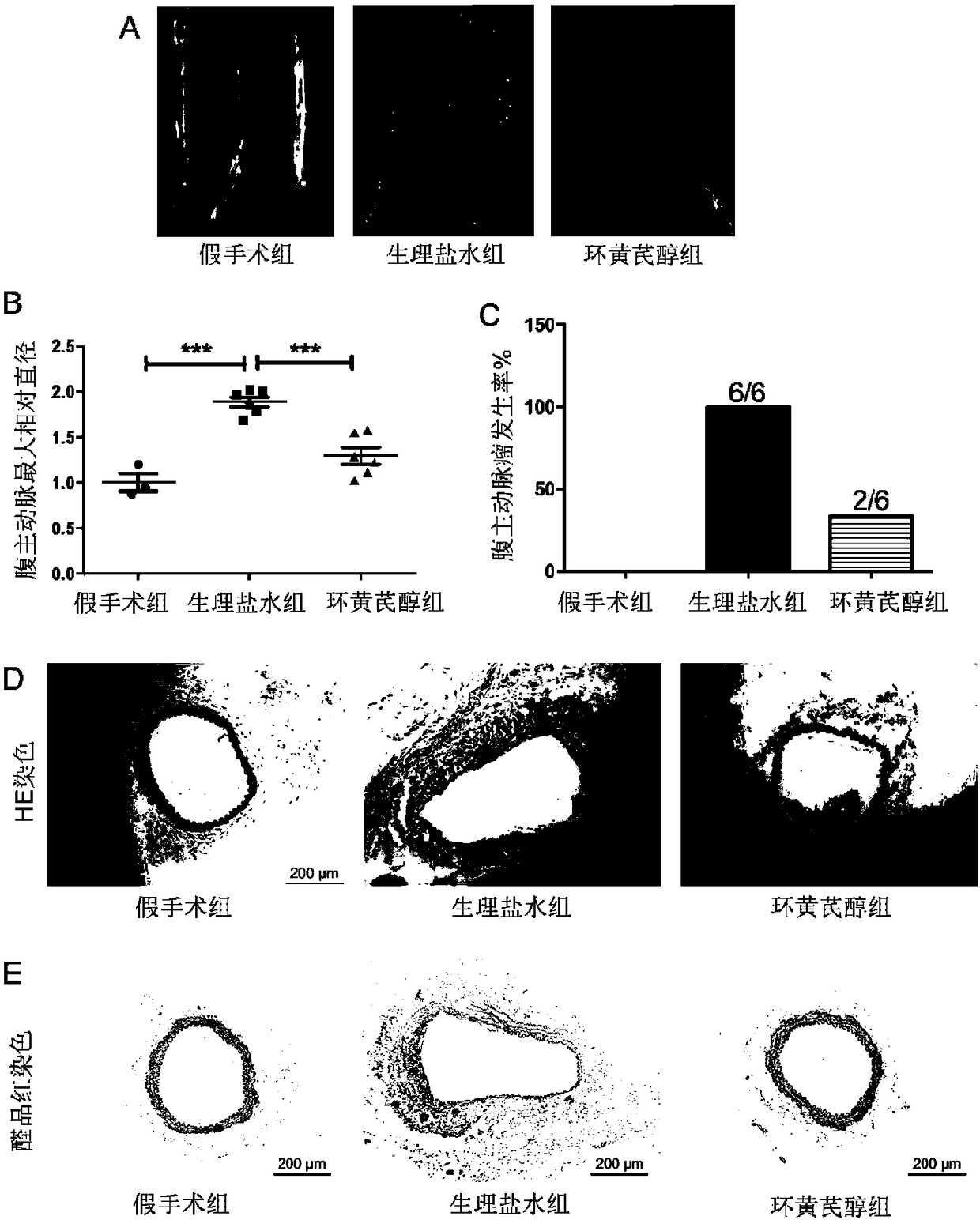
[0087] Embodiment 1, the application of cycloastragenol in the prevention of abdominal aortic aneurysm
[0088] 1. Mouse infrarenal aorta incubated with elastase to induce abdominal aortic aneurysm model
[0089] Experimental animals: 10-week-old male C57BL / 6J mice (average body weight 23 g).
[0090] The experimental animals were divided into model group and sham operation group (12 in the model group and 3 in the sham operation group).
[0091] 1. Experimental animals were anesthetized by intraperitoneal injection of 5% chloral hydrate (injection according to body weight, 8 μL / g).
[0092] 2. After completing the anesthesia in step 1, the infrarenal abdominal aorta was separated, and a section of the abdominal aorta (about 1 cm in length) with no branches on the anterior wall and side wall was taken below the plane of the renal artery, and fixed with threading marks. In the model group, gauze soaked with 2U elastase was wrapped around the exposed abdominal aorta. After 50 ...
Embodiment 2
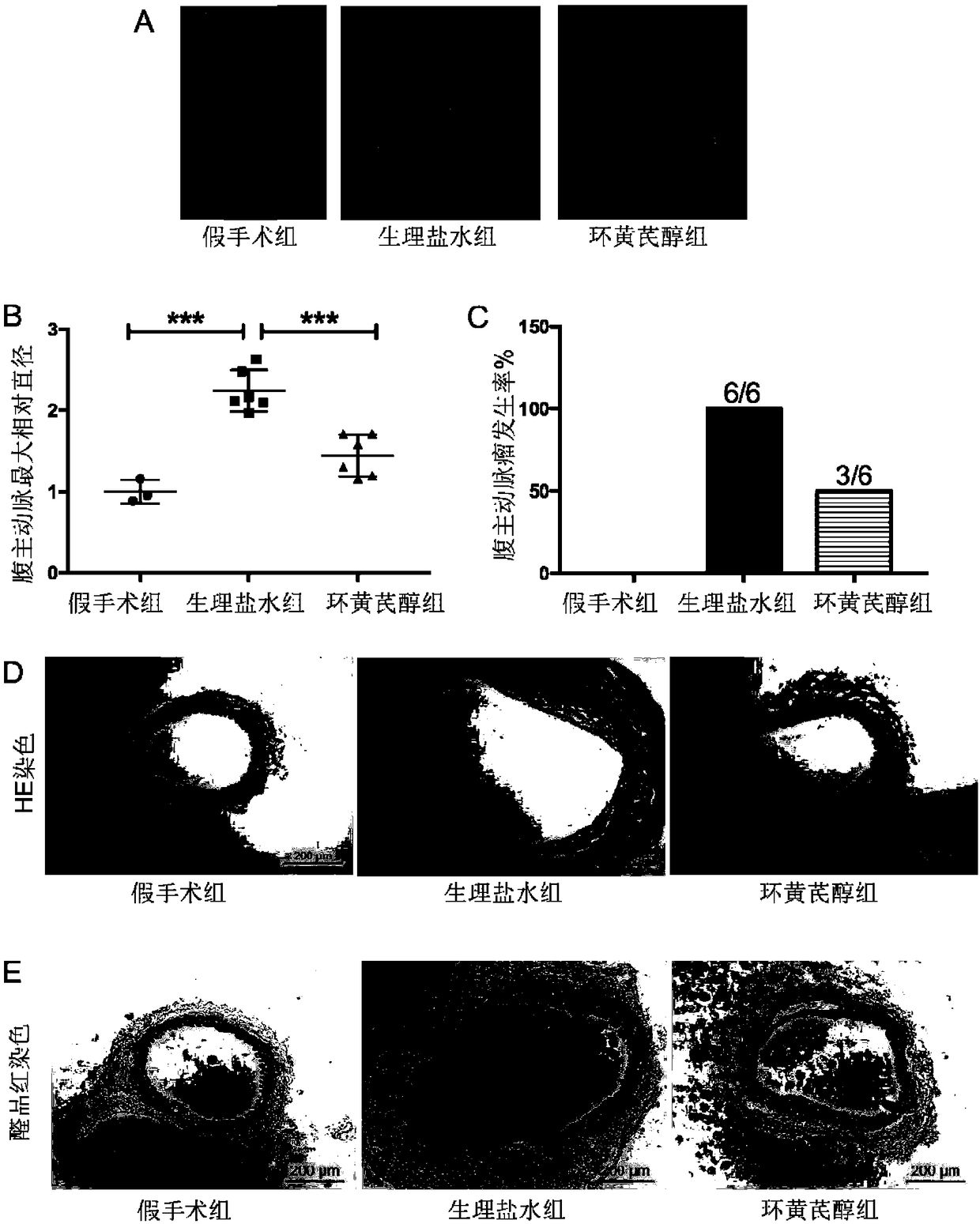
[0098] Example 2. Intragastric administration of cycloastragenol to treat abdominal aortic aneurysm induced by infrarenal artery of C57BL / 6J mice incubated with elastase
[0099] 1. Mouse infrarenal aorta incubated with elastase to induce abdominal aortic aneurysm model
[0100] Same as Step 1 of Example 1.
[0101] 2. Oral administration of cycloastragenol
[0102] 1. After 14 days of completing step 1, the mice in the model group were randomly divided into a normal saline group and a cycloastragenol group (6 mice in each group), and the cycloastragenol group mice were intragastrically administered with cycloastragenol every day (cycloastragelol dissolved In 0.01M PBS solution, the intragastric administration dose is 125mg cycloastragenol / kg body weight), the mice in the normal saline group were intragastrically administered with normal saline (200 μL / only), and the mice in the sham operation group were intragastrically administered with normal saline (200 μL / only). / piece)...
Embodiment 3
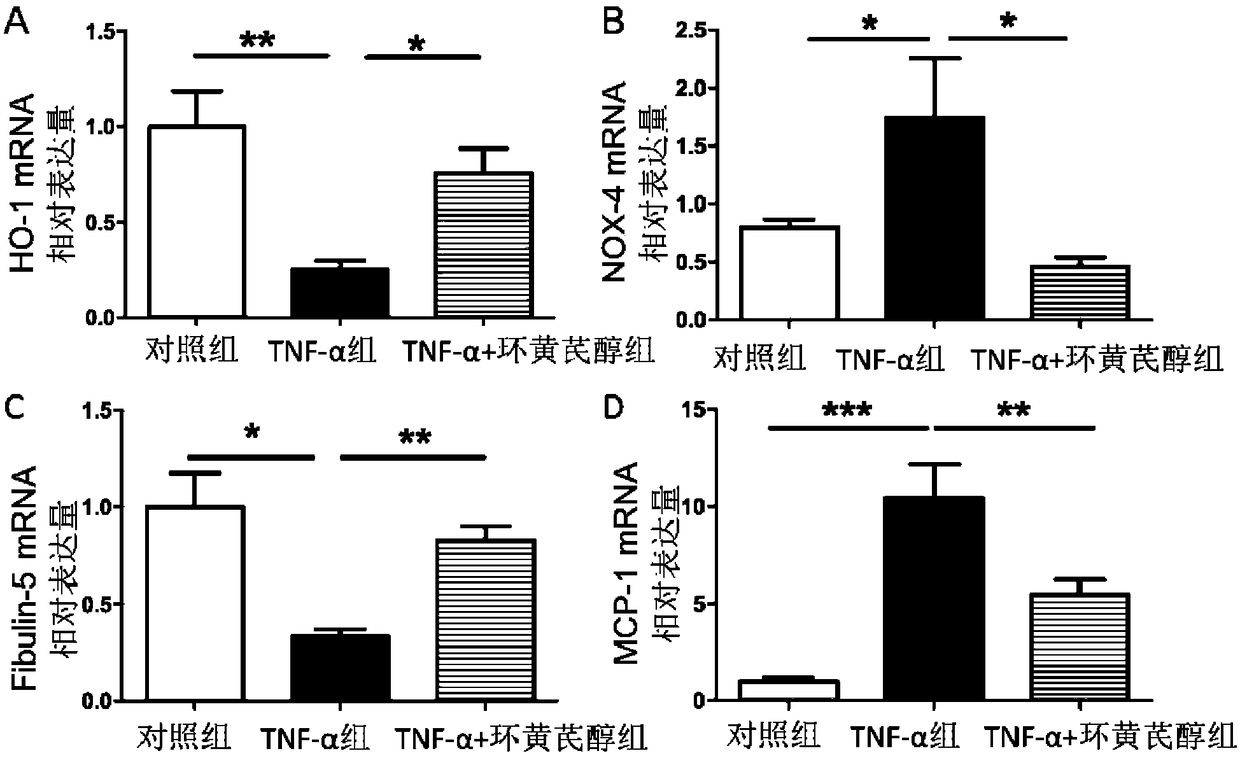
[0106] Example 3, cycloastragenol inhibits the injury of rat vascular smooth muscle cells induced by TNF-α
[0107] 1. Preparation of rat vascular smooth muscle cells
[0108] SD rats (body weight about 100 g) were anesthetized with 10% chloral hydrate, soaked in 75% alcohol for 2 min for disinfection. The chest cavity and abdominal cavity were opened, the organs were pushed aside, the heart and aorta were exposed, and the blood was sucked away with gauze. Carefully cut the full length of the heart and aorta up to the bifurcation of the abdominal aorta, and immediately put it in 0.01M PBS buffer. The adipose tissue and connective tissue were separated, and the heart was carefully cut off, during which the heart was washed three times with 0.01M PBS buffer. The separated transparent aorta was washed once in DMEM containing 0.05g / 100ml amphotericin B. The aorta was then cut longitudinally, the intima was scraped off, and the aorta was minced. Transfer the cut aortic tissue p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com