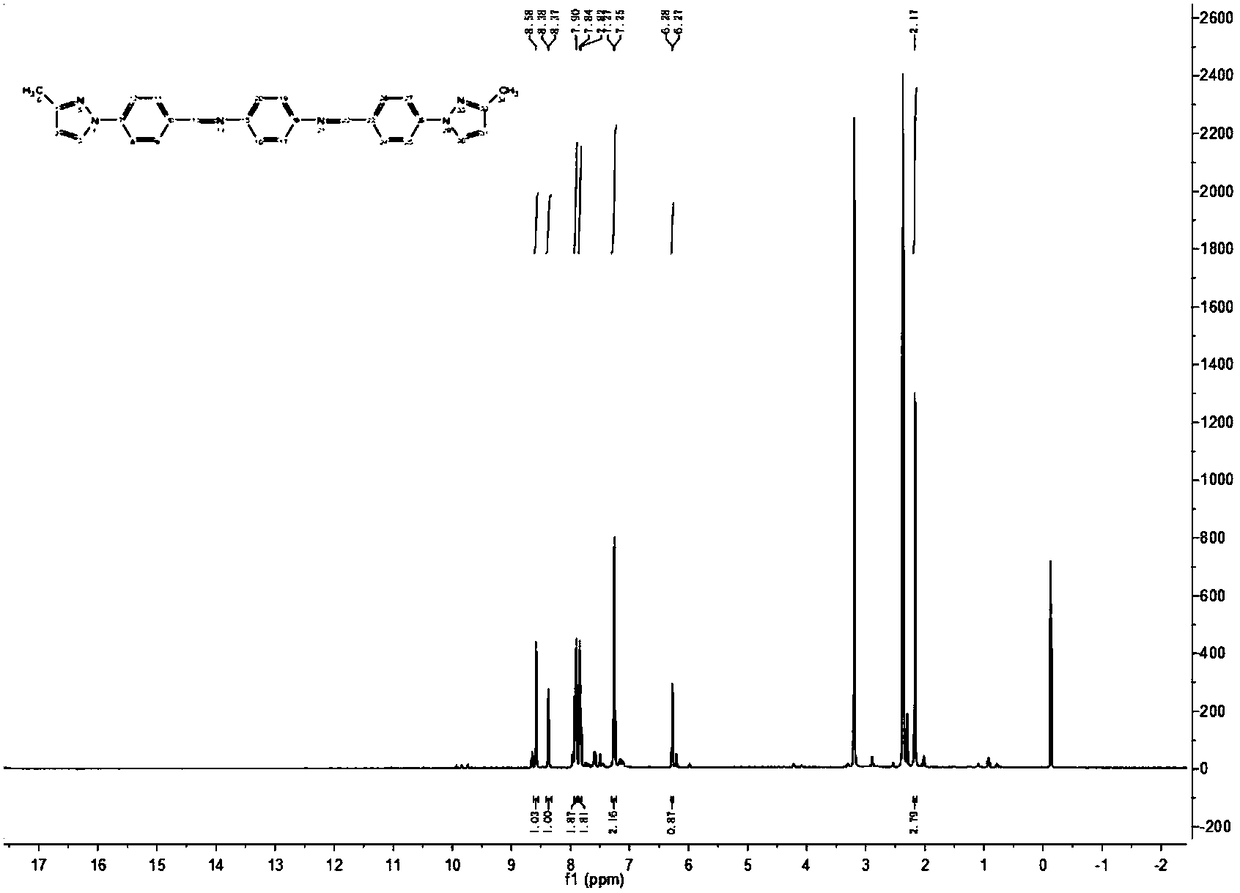
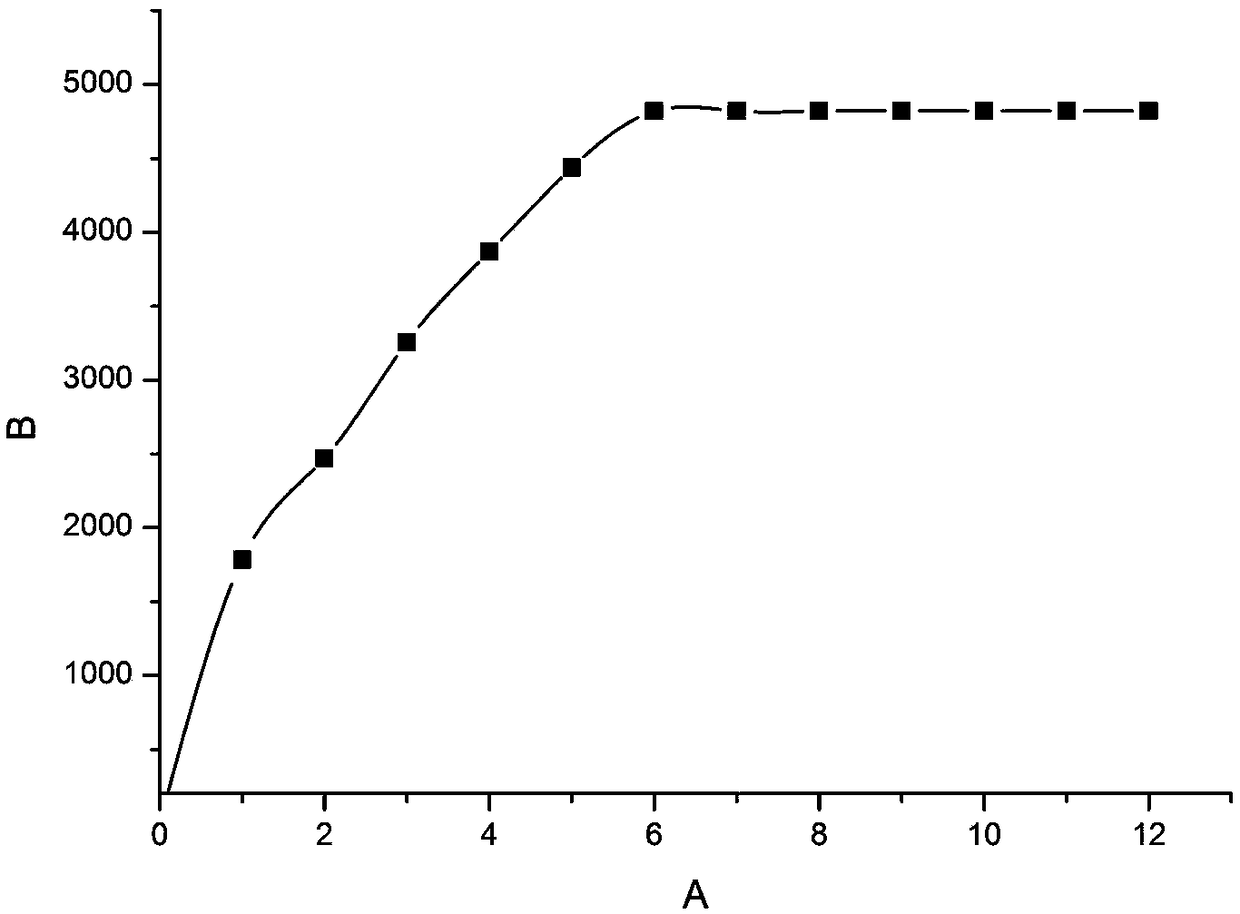
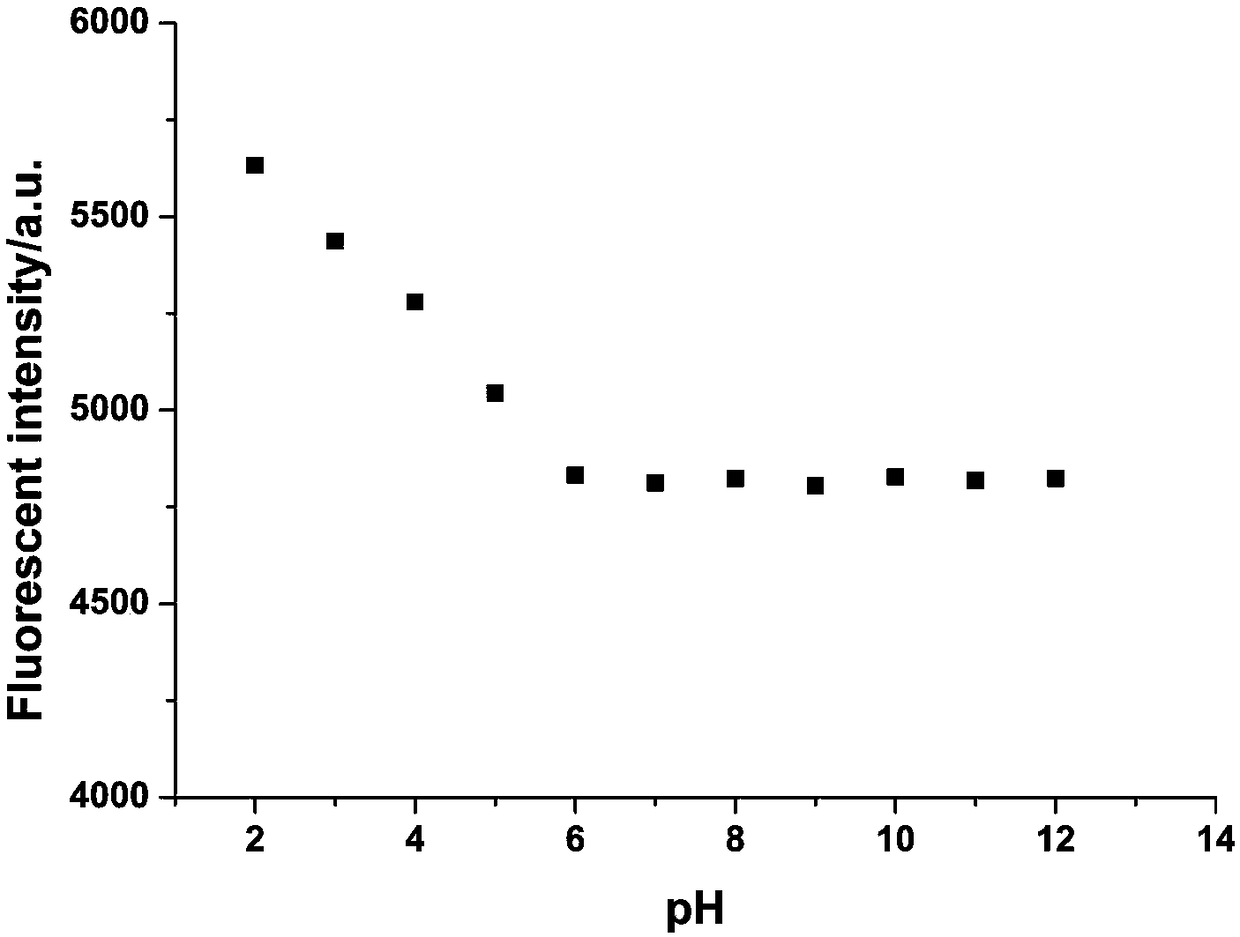
3-methylpyrazolyl benzaldehyde 4-aminoantipyrine Schiff base and preparation method thereof
A technology of aminoantipyrine and methylpyrazolyl, applied in the field of 3-methylpyrazolylbenzaldehyde acetal 4-aminoantipyrine Schiff base and its preparation, to achieve good specific selectivity, fast Response to the effect of pH adaptation range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 0.82g (10mmol) of 3-methylpyrazole and 2.32g (16.8mmol) of potassium carbonate, dissolve them in 20mL of N,N-dimethylformamide, and add them to a 100mL tetrachloride equipped with a thermometer and a stirring device. mouth flask. React under constant temperature stirring at 100°C, add 1.24g (10mmol) of p-fluorobenzaldehyde to the mixed solution, continue the reaction for 16h, cool to room temperature, filter, extract, separate by column chromatography, concentrate under reduced pressure, wash with warm water, and reconstitute with ether Crystallize and dry in vacuum at 50°C for 8 hours to obtain 3-methylpyrazolylbenzaldehyde.
[0031] Weigh 0.93g (5mmol) of 3-methylpyrazolylbenzaldehyde and 1.01g (5mmol) of 4-aminoantipyrine, dissolve them in 4mL of N,N-dimethylformamide, and add them to a thermometer equipped with a thermometer. , A 10mL single-necked flask with a stirring device. Add 0.12 g (2 mmol) of glacial acetic acid to the above mixed solution, react at ...
Embodiment 2
[0033] Weigh 0.82g (10mmol) of 3-methylpyrazole and 2.32g (16.8mmol) of potassium carbonate, dissolve them in 15mL of N,N-dimethylformamide, and add them to a 100mL tetrachloride equipped with a thermometer and a stirring device. mouth flask. React under constant temperature stirring at 110°C, add 3.1 g (25 mmol) of p-fluorobenzaldehyde to the mixed solution, continue the reaction for 16 h, cool to room temperature, filter, extract, separate by column chromatography, concentrate under reduced pressure, wash with warm water, and reconstitute with ether Crystallize and dry in vacuum at 50°C for 8 hours to obtain 3-methylpyrazolylbenzaldehyde.
[0034] Weigh 0.186g (1mmol) of 3-methylpyrazolylbenzaldehyde and 0.202g (1mmol) of 4-aminoantipyrine, dissolve them in 5mL of N,N-dimethylformamide, and add to the , A 10mL single-necked flask with a stirring device. Add 0.30 g (5 mmol) of glacial acetic acid to the above mixed solution, react at 85 °C for 4 h under constant temperature...
Embodiment 3
[0036] Weigh 1.64g (20mmol) of 3-methylpyrazole and 5.53g (40mmol) of potassium carbonate, dissolve them in 30mL of N,N-dimethylformamide, and add them to a 100mL four-port tube equipped with a thermometer and a stirring device. in the flask. React under constant temperature stirring at 120°C, add 1.61 g (13 mmol) of p-fluorobenzaldehyde to the mixed solution, continue the reaction for 18 h, cool to room temperature, filter, extract, separate by column chromatography, concentrate under reduced pressure, wash with warm water, and reconstitute with ether Crystallize and dry in vacuum at 50°C for 8 hours to obtain 3-methylpyrazolylbenzaldehyde.
[0037]Weigh 0.186g (1mmol) of 3-methylpyrazolylbenzaldehyde and 1.01g (0.5mmol) of 4-aminoantipyrine, dissolve them in 4mL of N,N-dimethylformamide, add to the In a 10mL single-necked flask with a thermometer and a stirring device. Add 0.12 g (2 mmol) of glacial acetic acid to the above mixed solution, react at 80 °C for 4 h under cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com