Fluorescent protein, fusion protein, isolated nucleic acid, vector and application thereof
A fluorescent protein and nucleic acid technology, applied in the field of biomedical optics and molecular imaging, can solve the problems of difficult to achieve simultaneous imaging of multiple fluorescent proteins, difficult to achieve synchronous control, and high cost, to reduce crosstalk, reduce high requirements, and improve The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
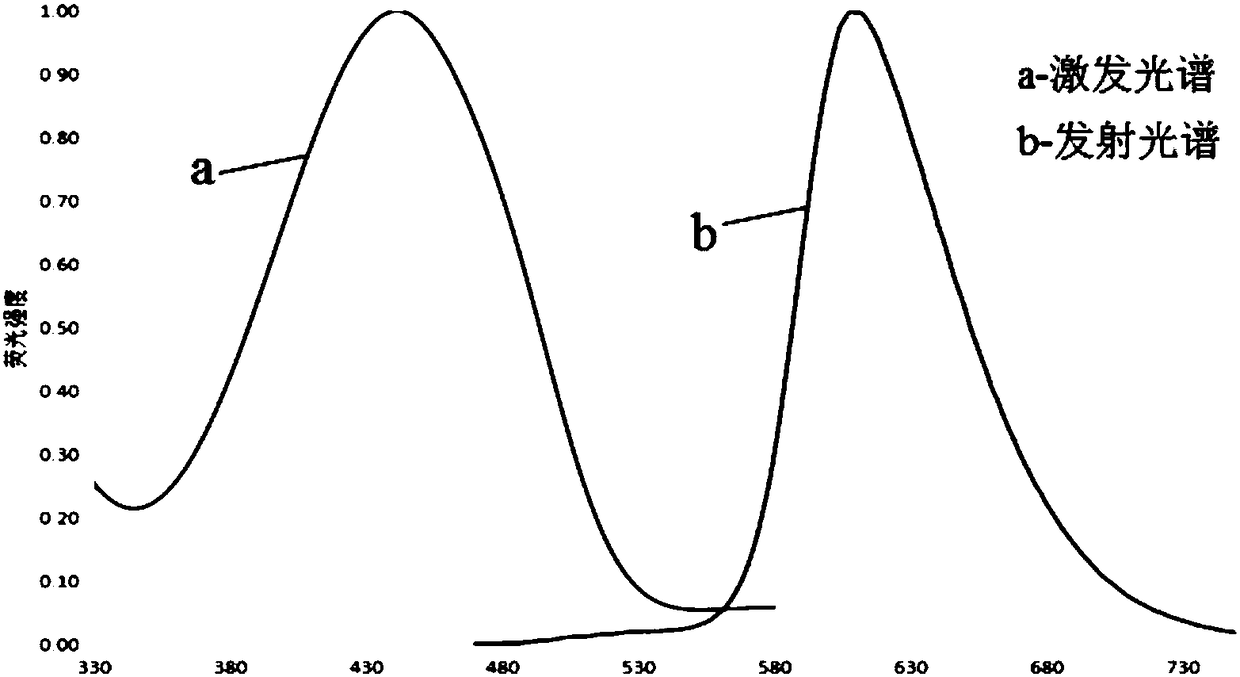
[0060] The amino acid sequence of the fluorescent protein provided in this example is shown in SEQ ID NO: 2, which includes the following amino acid mutation site: C158D relative to the fluorescent protein mCrimson (whose amino acid sequence is shown in SEQ ID NO: 1).
[0061] The mutation site C158D, the cysteine at position 158 of the mCrimson amino acid sequence is replaced by aspartic acid, which changes the fluorescence spectrum properties of the fluorescent protein of the present invention and has a larger Stokes shift characteristic , its excitation peak and emission peak are 451nm and 613nm respectively, therefore, this fluorescent protein can be used together with the existing fluorescent protein column such as green fluorescent protein, can be used in single photon or multiphoton microscopic multicolor imaging, can effectively Reducing the crosstalk between the fluorescence spectra of different fluorescent proteins helps to improve the sensitivity of imaging, ensure...
Embodiment 2
[0063] The amino acid sequence of the fluorescent protein provided in this example is shown in SEQ ID NO: 3, which includes the following amino acid mutation sites relative to the fluorescent protein mCrimson: C158D, M61F and F174I.
[0064] The fluorescent protein provided in this embodiment not only has the same effect as the fluorescent protein in Example 1, but also through the introduction of M61F and F174I mutation sites, these two mutation sites reflect the brightness characteristics of the fluorescent protein, making the fluorescent protein provided by the present invention Fluorescent proteins also have higher brightness due to their large Stokes shift.
Embodiment 3
[0066] The fluorescent protein provided in this example has an amino acid sequence as shown in SEQ ID NO: 4, which includes the following amino acid mutation sites relative to the fluorescent protein mCrimson: C158D, R10T, Q23Y, G75D, Q111R, E114V, L121V, Q135R and Y218H .
[0067] The fluorescent protein provided in this example not only has the same effect as the fluorescent protein in Example 1, but also through the introduction of multiple mutation sites R10T, Q23Y, G75D, Q111R, E114V, L121V, Q135R and Y218H, these sites reflect the The maturation rate characteristic of the fluorescent protein makes the fluorescent protein provided by the present invention have a higher maturation rate in terms of the characteristic of a large Stokes shift.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com