Use of linc01836 in the preparation of gastric cancer diagnostic products and therapeutic drugs
A gastric cancer and product technology, applied in the field of biomedicine, can solve the problems of non-conservation of lncRNA, diagnosis and gene therapy impact, etc., to achieve the effect of improving early diagnosis and reducing mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Screening of differentially expressed non-long-chain coding RNA
[0052] 1. Research object:
[0053] Surgical specimens (gastric cancer tissues and corresponding paracancerous tissues) of 5 patients with primary gastric cancer who underwent radical gastrectomy in the Department of Oncology of the hospital were collected, and each patient signed an informed consent.
[0054] Inclusion criteria: a. Preoperative diagnosis of primary gastric cancer, and no tumor treatment; b. No other malignant tumors; c. No other complications, such as gastric perforation, gastrointestinal bleeding, gastrointestinal obstruction, etc. The general condition of the patient was good before operation; d. No other chronic diseases, such as hypertension, diabetes, etc.
[0055] This study was approved by the ethics committee.
[0056] 2. Sample acquisition
[0057] 1) After the surgical sample is isolated from the tumor, the tumor sample is selected, with a size of about 0.5cm x 0.5...
Embodiment 2
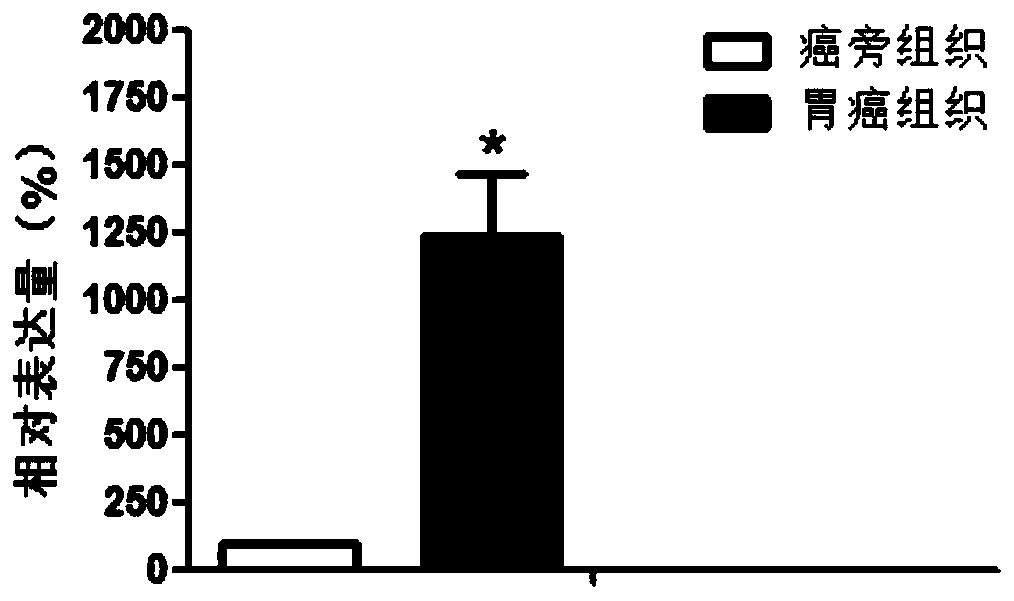
[0085] Example 2 Large sample verification screened out differentially expressed LncRNA
[0086] Based on the screening results of Example 1 and according to the size of P value, LINC01836 was selected for verification.
[0087] 1. Sample collection
[0088] According to the method of Example 1, 45 gastric cancer tissues and 45 corresponding paracancerous tissues were collected.
[0089] 2. Validation at the transcript level
[0090] Reagents: Reverse transcription kit (DDR037A) was purchased from Treasure Bioengineering (Dalian) Co., Ltd. SYBR Premix Ex Taq for real-time quantitative PCR (polymerase chain reaction) TM (Tli RNaseHPlus) kit was produced by Japan Takara Company.
[0091] 2.1 Extract tissue RNA
[0092] Step is with embodiment 1.
[0093] 2.2 Primer design
[0094] According to the LINC01836 transcript sequence, primers were designed by NCBI's primer design tool (Primer BLAST), upstream primer: 5'-TGAAGAAACCGTGGAAAC-3' (SEQ ID NO.2); downstream primer: 5'-...
Embodiment 3
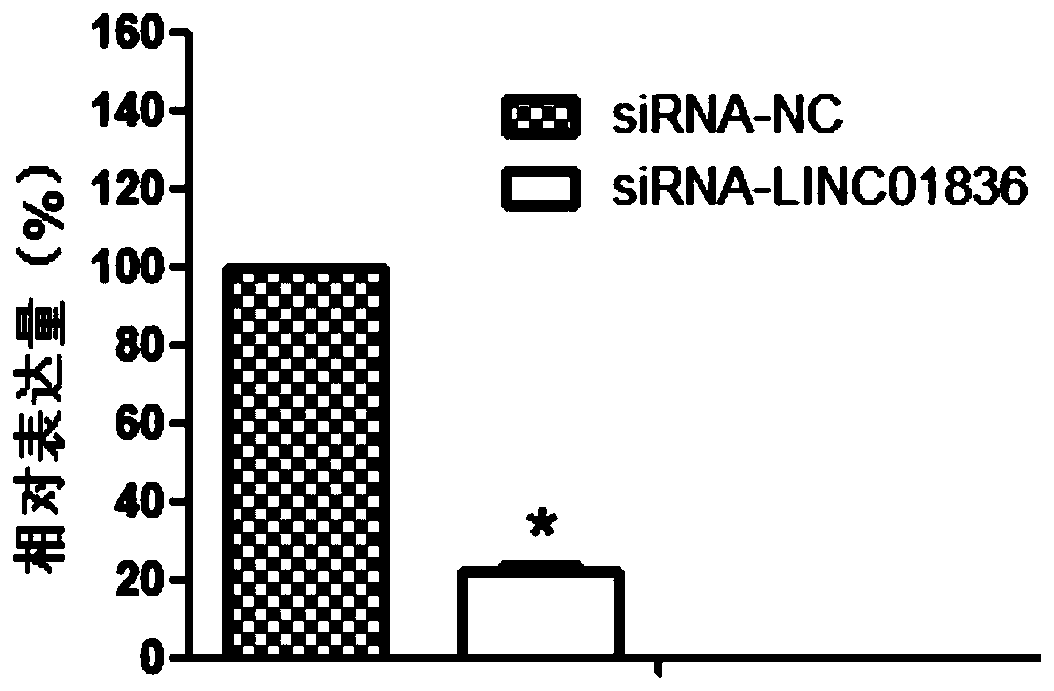
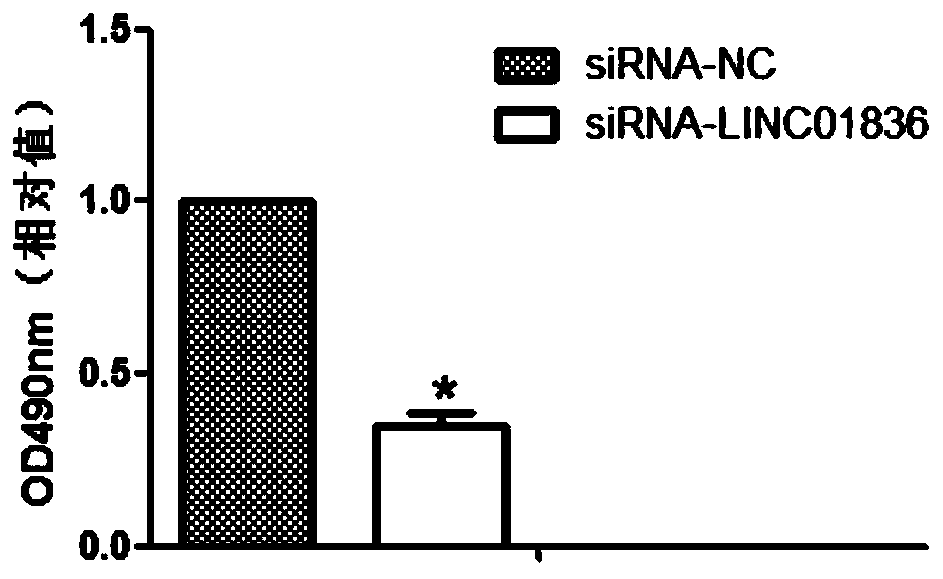
[0102] Embodiment 3 inhibits the expression of LINC01836
[0103] 1. Cell culture and transfection
[0104] Cell culture: Gastric cancer cell line BGC-823 was used in DMEM containing 10% FBS and placed in 5% CO 2 , Saturated humidity, cultured in a 37°C carbon dioxide incubator. The culture medium was changed every two days, and the cells were digested with 0.25% trypsin when subcultured.
[0105] siRNA transfection: The day before transfection, cells are digested and inoculated into culture dishes or culture plates, and the number of cells inoculated should ensure that the density of 30-50% can be reached on the second day of transfection. siRNA transfection strictly according to Lipofectamin TM 2000 instructions, after 4-6 hours, replace with fresh culture medium containing 10% FBS, and continue to cultivate for 48-72 hours.
[0106] 2. siRNA design
[0107] Design of siRNA (small interfering RNA): siRNA sequence was designed in the specific sequence region of LINC01836...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com