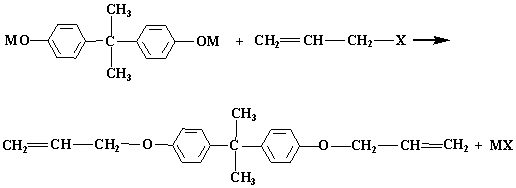
Preparation method of bisphenol A diallyl ether
A technology of bisallyl ether and allyl halide, applied in the field of organic compound synthesis, can solve the problems of influence of purity, deep color and the like, and achieve the effects of low price, simple reaction conditions and production cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
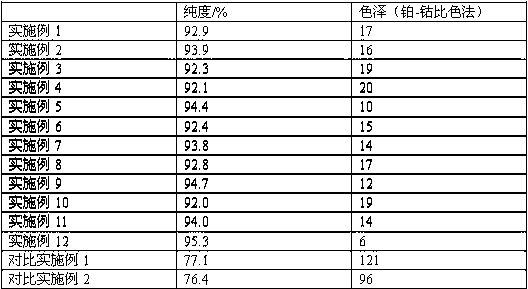
[0030] Add 228g of bisphenol A into a 1000ml four-neck flask, start stirring, add 260g of toluene, after the bisphenol A is completely dissolved, add 90g of sodium hydroxide and 28.9g of calcium oxide, stir evenly, start to drop 229.5g of allyl chloride, The temperature was raised to 100°C, and the reaction was kept for 3 hours. After the reaction was completed, the temperature was lowered to room temperature to obtain a crude product; the crude product was filtered to remove salt and vacuum distillation to remove low boilers with a purity of 92.9% and a color of 17.
Embodiment 2
[0032] Add 228g of bisphenol A into a 1000ml four-neck flask, start stirring, add 260g of xylene, after the bisphenol A is completely dissolved, add 123.2g of potassium hydroxide and 30.6g of calcium oxide, stir evenly, and start to dropwise add 229.5g of allyl chloride g, the temperature was raised to 100° C., and the temperature was kept for 3 hours. After the reaction was completed, the temperature was lowered to room temperature to obtain a crude product; the crude product was filtered to remove salt and vacuum distillation to remove low boilers, and the product obtained had a purity of 93.9% and a color of 16.
Embodiment 3
[0034] Add 228g of bisphenol A into a 1000ml four-neck flask, start stirring, add 450g of ethylene glycol dimethyl ether, after the bisphenol A is completely dissolved, add 80g of sodium hydroxide and 100g of anhydrous calcium sulfate, stir evenly, and start to add ethylene glycol dimethyl ether dropwise. 382.5g of propyl chloride was heated up to 85°C and kept for 16h. After the reaction was completed, the temperature was lowered to room temperature to obtain a crude product; the crude product was filtered to remove salt and vacuum distillation to remove low boilers, and the product obtained had a purity of 92.3% and a color of 19.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| color | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com