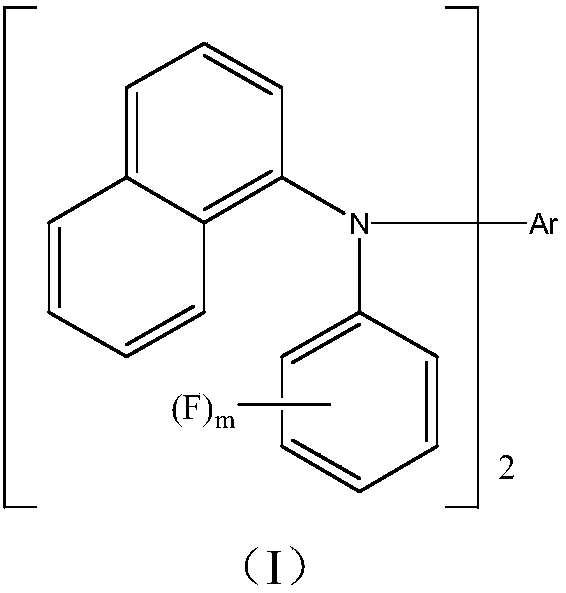
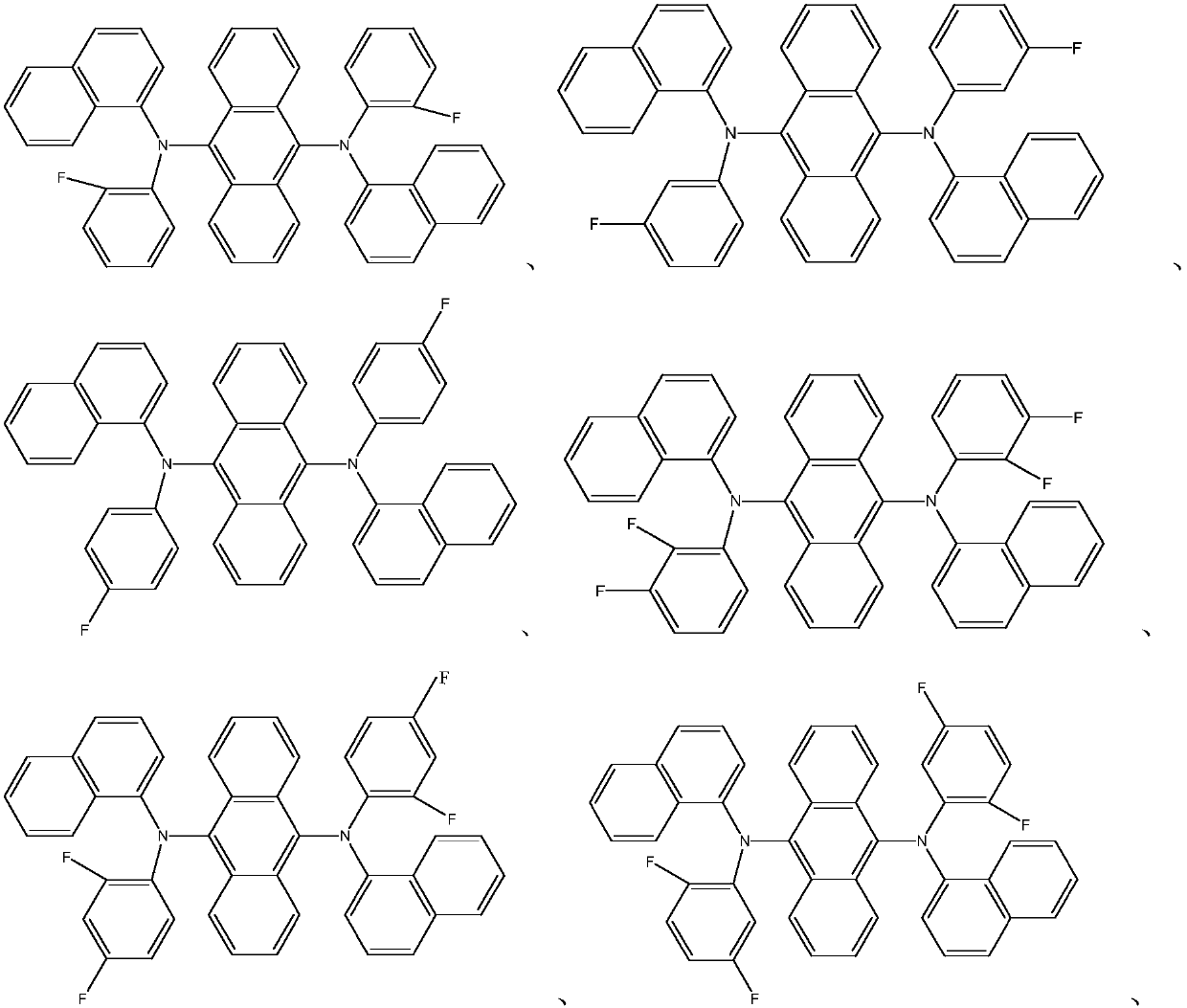
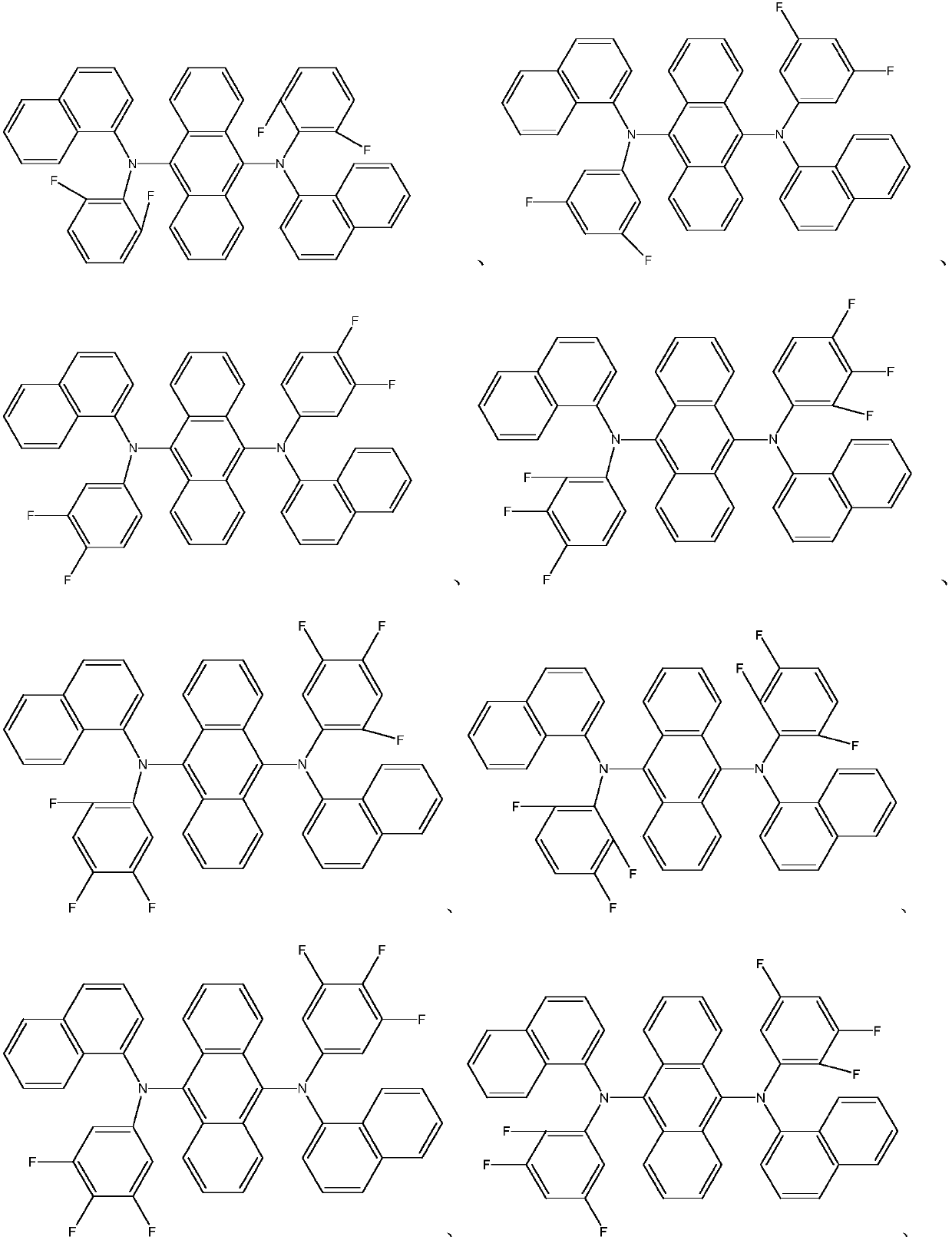
Novel organic material and synthesis and application of novel organic material
An organic material and a new type of technology, applied in the field of organic electroluminescence display, can solve the problems of affecting the service life of materials, damage to the uniformity of the film, low glass transition temperature, etc. The effect of mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of (Compound 1)
[0067] The synthetic route is as follows:
[0068]
[0069] 1) Synthesis of compound 1-1
[0070]A 1000 ml three-necked flask was equipped with a magnetic stirrer, and 46.1 g (0.48 mol) of sodium tert-butoxide, 33.3 g (purity 99%, 0.3 mol), 41.4 g 1-bromonaphthalene (purity 99%, 0.3 mol), 41.4 g 1-bromonaphthalene (purity 99%, 0.2mol) and toluene 400ml. After argon replacement again, 3 ml of tri-tert-butylphosphine and 0.46 g of tris(diphenylbenzylacetone)dipalladium were added in sequence. After the addition, start stirring and heat up to 100°C, and control the temperature at 100-110°C for 5 hours. After cooling down to 30°C, the filtrate was obtained by suction filtration through a silica gel column, and the filtrate was rotary evaporated, dissolved in dichloromethane, washed twice with 4mol / L hydrochloric acid solution, separated, dried with anhydrous sodium sulfate, suction filtered, and rotary evaporated The filtrate obtained 44...
Embodiment 2
[0075] Synthesis of (Compound 2)
[0076] The synthetic route is as follows:
[0077]
[0078] 1) Synthesis of compound 2-1
[0079] A 1000 ml three-necked flask was equipped with a magnetic stirrer, and 46.1 g (0.48 mol) of sodium tert-butoxide, 33.3 g (purity 99%, 0.3 mol), 41.4 g 1-bromonaphthalene (purity 99%, 0.3 mol), 41.4 g 1-bromonaphthalene (purity 99%, 0.2mol) and toluene 400ml. After argon replacement again, 3 ml of tri-tert-butylphosphine and 0.46 g of tris(diphenylbenzylacetone)dipalladium were added in sequence. After the addition, start stirring and heat up to 100°C, and control the temperature at 100-110°C for 5 hours. After cooling down to 30°C, the filtrate was obtained by suction filtration through a silica gel column, and the filtrate was rotary evaporated, dissolved in dichloromethane, washed twice with 4mol / L hydrochloric acid solution, separated, dried with anhydrous sodium sulfate, suction filtered, and rotary evaporated The filtrate obtained 4...
Embodiment 3
[0084] Synthesis of (Compound 3)
[0085] The synthetic route is as follows:
[0086]
[0087] 1) Synthesis of compound 3-1
[0088] A 1000 ml three-necked flask was equipped with a magnetic stirrer, and 46.1 g (0.48 mol) of sodium tert-butoxide, 33.3 g (purity 99%, 0.3 mol), 41.4 g 1-bromonaphthalene (purity 99%, 0.3 mol), 41.4 g 1-bromonaphthalene (purity 99%, 0.2mol) and toluene 400ml. After argon replacement again, 3 ml of tri-tert-butylphosphine and 0.46 g of tris(diphenylbenzylacetone)dipalladium were added in sequence. After the addition, start stirring and heat up to 100°C, and control the temperature at 100-110°C for 5 hours. After cooling down to 30°C, the filtrate was obtained by suction filtration through a silica gel column, and the filtrate was rotary evaporated, dissolved in dichloromethane, washed twice with 4mol / L hydrochloric acid solution, separated, dried with anhydrous sodium sulfate, suction filtered, and rotary evaporated The filtrate obtained 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com