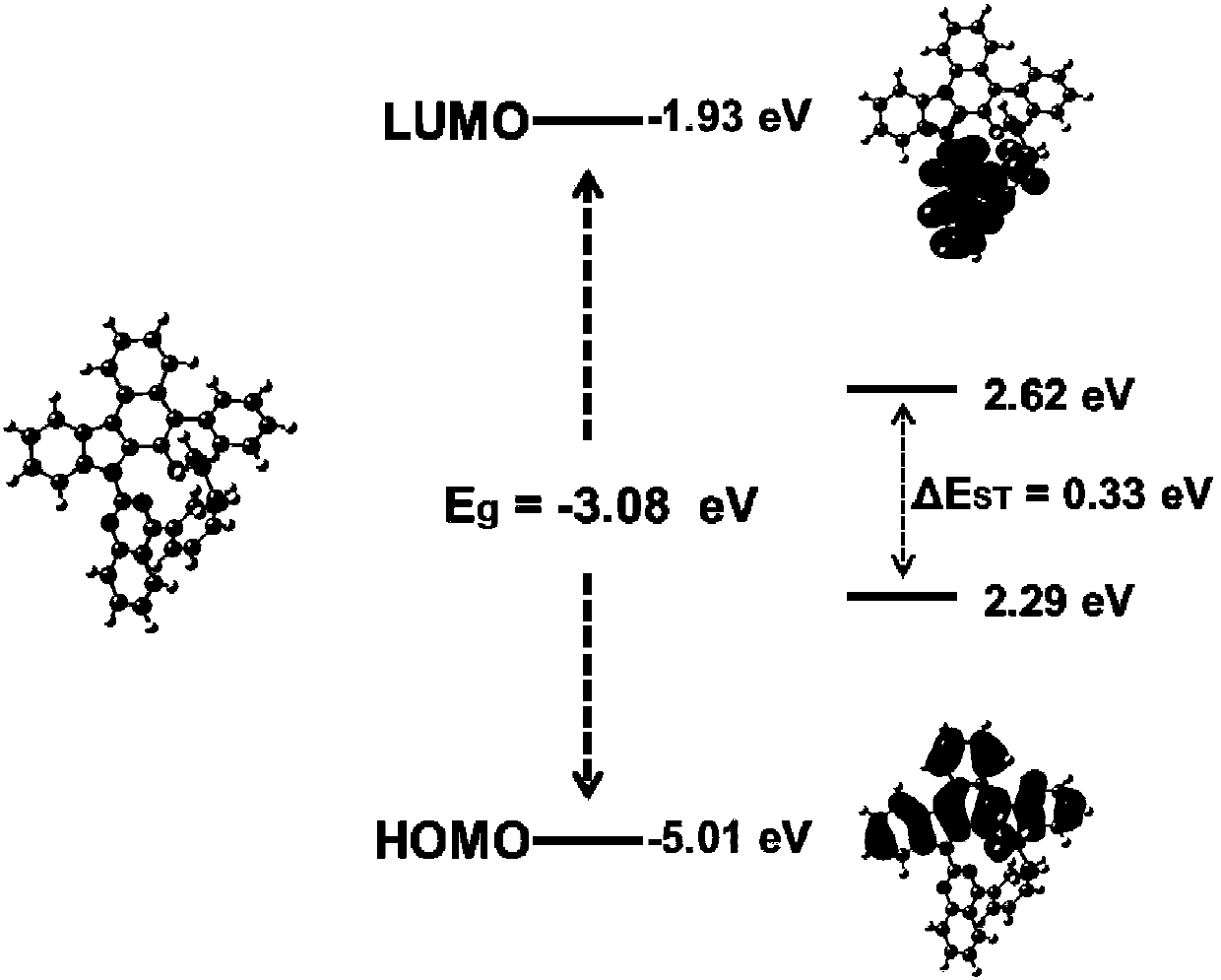
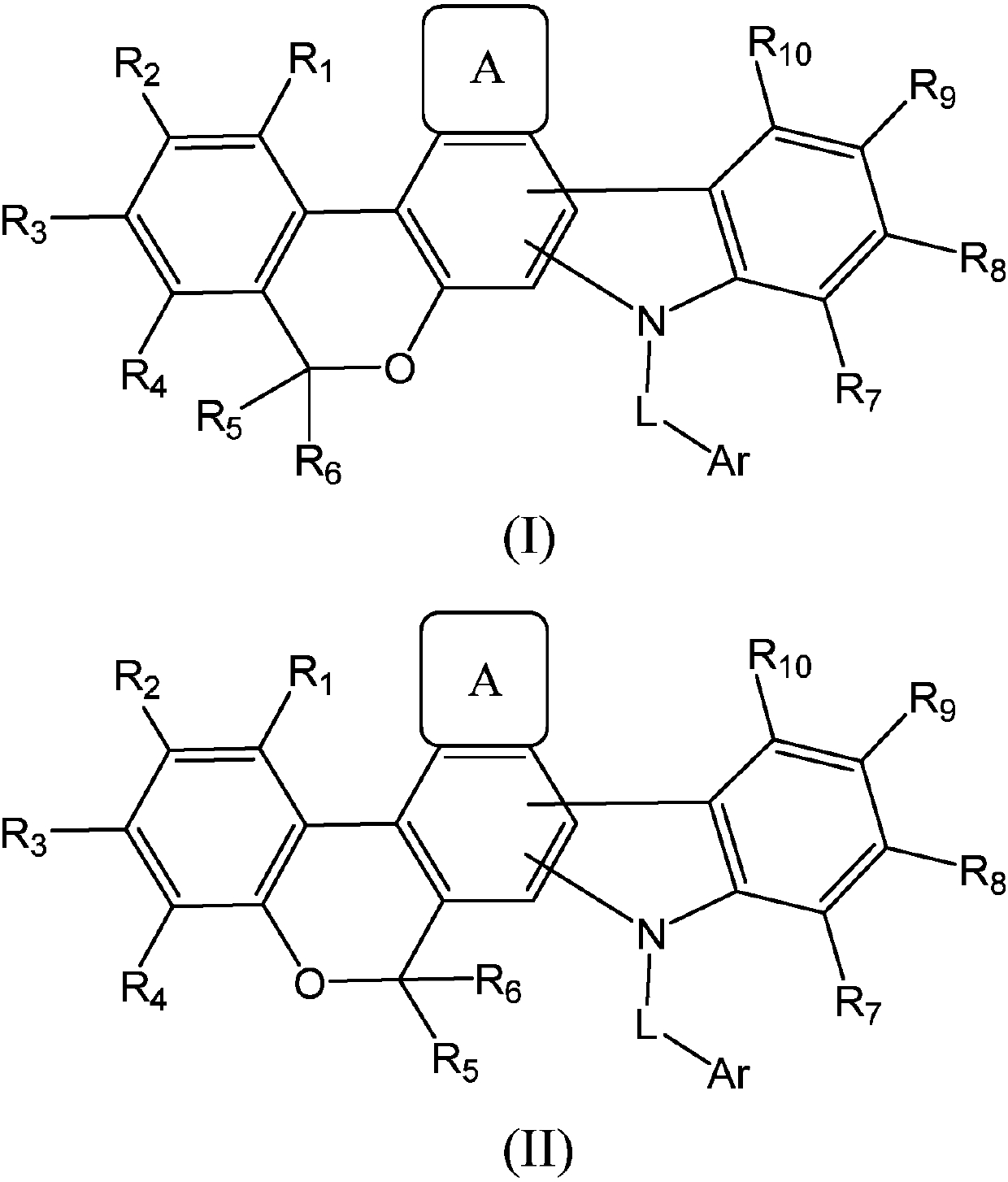
Fused polycyclic compound as well as preparation method and application thereof
A technology of condensed polycyclic compounds, applied in chemical instruments and methods, organic chemistry, semiconductor/solid-state device manufacturing, etc. Low problems, to achieve the effect of improving performance and luminous efficiency, high thermal stability and morphological stability, and improved matching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] This embodiment provides a fused polycyclic compound having the structure shown in the following formula D-5:
[0064]
[0065] The synthetic route of fused polycyclic compound shown in formula D-5 is as follows:
[0066]
[0067] The preparation method of the fused polycyclic compound shown in formula D-5 specifically comprises the following steps:
[0068] (1) Synthesis of Intermediate 1-1
[0069] Under nitrogen protection, in a 500mL two-necked reaction flask, add 1.6g of lithium hydroxide, add 100mL of N-methylpyrrolidone and water, add 7.44g of the compound shown in formula (A-1) and 7.6g of formula (C-1 ) shown in the compound, adding people triacetylacetonate dippalladium chloroform adduct under stirring. Stirring was then continued for 18 hours at 65°C. After the reaction was completed, hydrochloric acid was added to acidify, extracted with methyl tert-butyl ether, and after the organic liquid phase was distilled off, 6.8g of solid intermediate 1-1 was...
Embodiment 2
[0082] This embodiment provides a fused polycyclic compound having the structure shown in the following formula D-4:
[0083]
[0084] The synthetic route of condensed polycyclic compound shown in formula D-4 is as follows:
[0085]
[0086] The preparation method of the fused polycyclic compound shown in formula D-4 specifically comprises the following steps:
[0087] (1) By the synthesis method shown in Example 1, intermediate 5-1 was synthesized.
[0088] (2) Synthesis of fused polycyclic compound D-4
[0089] Under nitrogen protection, add 1.2g intermediate 5-1 (3.34mmol), 22mg palladium acetate (0.1mmol), 74mg tri-tert-butylphosphine (0.4mmol), 1.32g compound (3.34mmol), 0.9g sodium tert-butoxide, 50mL toluene, mix well, react at 110°C for 12 hours, cool to room temperature, extract with chloroform, remove solvent by rotary evaporation, and pass through a silica gel column to obtain 1.8g solid fused polycyclic compound D -4 (81% yield).
[0090] Elemental analy...
Embodiment 3
[0092] This embodiment provides a fused polycyclic compound having the structure shown in the following formula D-12:
[0093]
[0094] The synthetic route of condensed polycyclic compound shown in formula D-12 is as follows:
[0095]
[0096] (1) Synthesis of intermediate 1-2
[0097] Under nitrogen protection, into a 500mL two-necked reaction flask, add 1.6g of lithium hydroxide, add 100mL of N-methylpyrrolidone and water, add 7.44g of the compound shown in formula (A-1) and 7.6g of formula (C-2 ) shown in the compound, adding people triacetylacetonate dippalladium chloroform adduct under stirring. Stirring was then continued for 18 hours at 65°C. After the reaction was completed, hydrochloric acid was added to acidify, extracted with methyl tert-butyl ether, and the organic liquid phase was distilled off, and passed through a silica gel column to obtain 6.8 g of solid intermediate 1-2 (yield: 70%).
[0098] (2) Synthesis of Intermediate 2-2
[0099] Under the prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com