Novel nanometer drug and preparation method thereof
A nano-drug and nano-particle technology, used in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of low drug-carrying capacity and drug-carrying efficiency of carriers, affecting biological functions, and difficult to accurately control, and achieve a therapeutic approach. Synergy, guaranteed safety and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] figure 1 A flow chart showing a process for preparing self-assembled nanomedicines according to an embodiment of the present invention. Such as figure 1 As shown, first, a drug solution is prepared. Specifically, the hydrophobic drug is dissolved in dimethyl sulfoxide to obtain a first solution. Dissolve indocyanine green in water or NaHCO 3 aqueous solution to obtain a second solution.
[0054] In the first solution, the concentration of the hydrophobic drug may be 0.5-2 g / L.
[0055] In the second solution, the concentration of indocyanine green may be 0.2-1 g / L. In the second solution, if NaHCO is used 3 aqueous solution as solvent, NaHCO 3 In aqueous solution, NaHCO 3 The concentration can be 0.02 ~ 5mM.
[0056] Then, the drugs are homogeneously mixed and self-assembled to form nanomedicines. Specifically, the first solution and the second solution are mixed. The mixing process of the two is the process of self-assembly to form nano-medicines.
[0057] ...
Embodiment 1
[0067] Dissolve 1 mg of paclitaxel in 0.1 mL of DMSO to make a 10 mg / mL organic solution; make 1 mg / mL of ICG in NaHCO 3 (0.05mM) aqueous solution; under ultrasonic vibration, 0.1mL paclitaxel DMSO solution was added dropwise to 0.6mL ICG solution at a rate of 20μL every 15s, so that the solution was mixed evenly and self-assembled rapidly to form nano-drugs; centrifugal collection (17000rpm, 30min) to obtain the precipitation of drug nanoparticles; wash with PBS, and finally evenly disperse in 1mL of LPBS.
[0068] figure 2 It is the TEM image of the paclitaxel-ICG self-assembled nano-medicine synthesized in Example 1, which visually shows regular spherical morphology, uniform particle size and high dispersion.
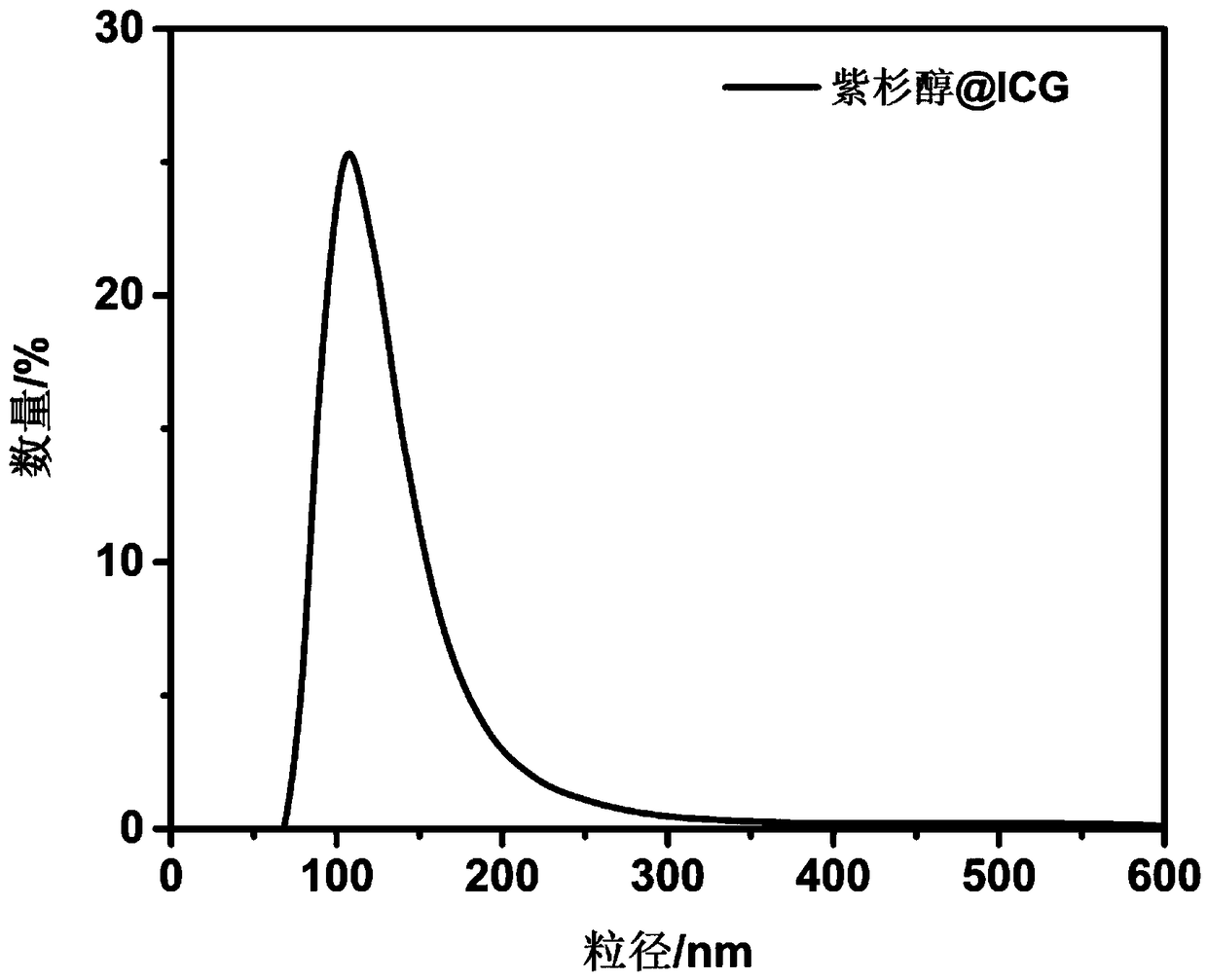
[0069] Figure 10 The particle size distribution diagram of the paclitaxel-ICG self-assembled drug synthesized in Example 1 is shown. It can be seen that the hydration kinetic diameter of the paclitaxel-ICG self-assembled nano-drug is about 100nm, with a single pe...
Embodiment 2
[0072] Dissolve 1 mg camptothecin in 0.1 mL DMSO to prepare a 10 mg / mL organic solution; prepare 1 mg / mL ICG in NaHCO 3 (0.05mM) aqueous solution; under ultrasonic vibration, the DMSO solution of 0.1mL camptothecin was added dropwise to the ICG solution of 0.6mL at a rate of 20 μL every 15s, so that the solution was mixed evenly and self-assembled rapidly to form nano-medicines; Collect by centrifugation (17000rpm, 30min) to obtain the precipitate of drug nanoparticles; wash with PBS, and finally evenly disperse in 1mL of PBS.
[0073] image 3 It is a TEM image of the camptothecin-ICG self-assembled nanomedicine synthesized in Example 2, which visually shows regular spherical morphology, uniform particle size and high dispersion.
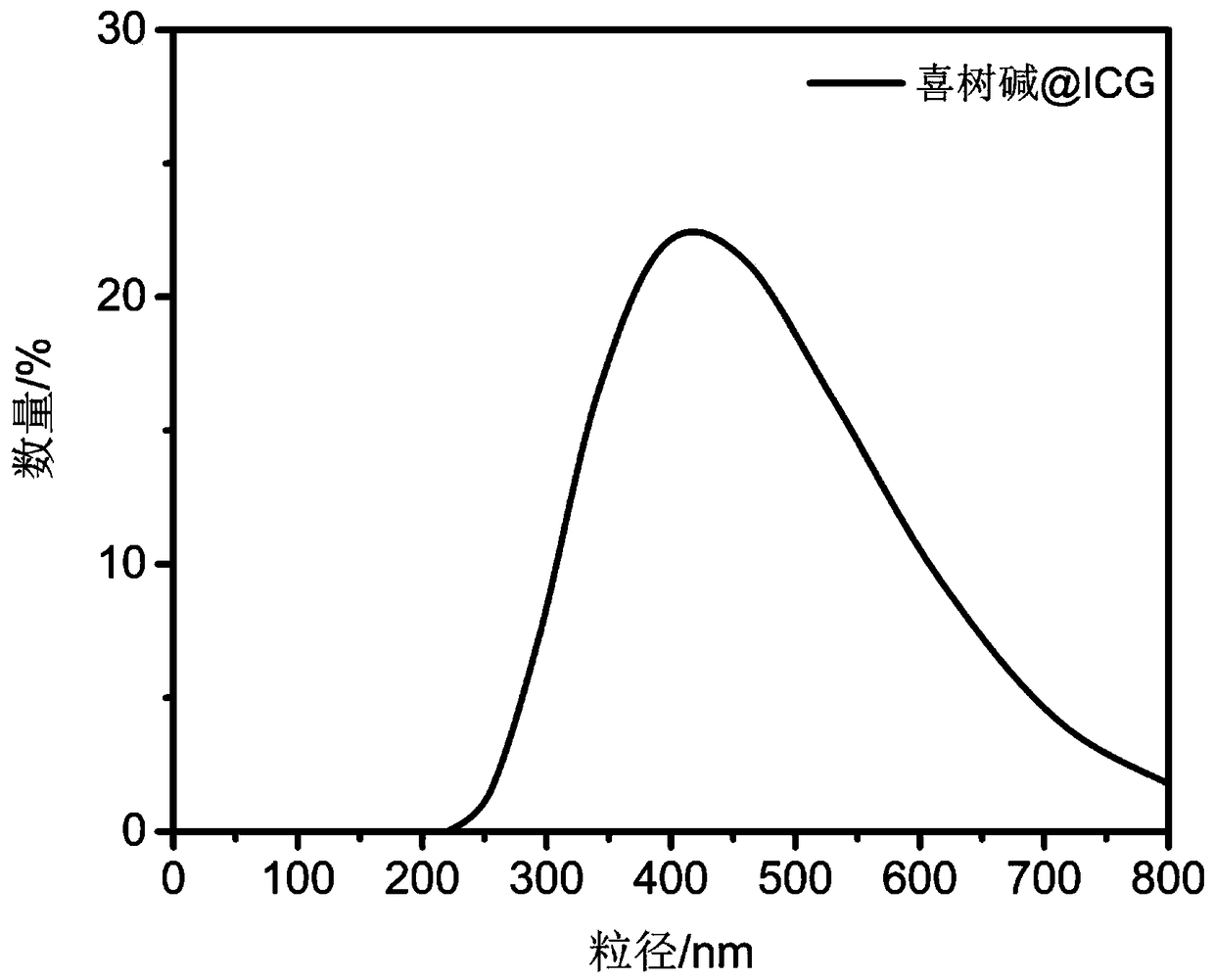
[0074] Figure 11 The particle size distribution diagram of the camptothecin-ICG self-assembled drug synthesized in Example 2 is shown. It can be seen that the hydration kinetic diameter of the camptothecin-ICG self-assembled nano drug is about 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com