A real-time fluorescent quantitative PCR primer, kit and method for detecting lentivirus virus titer
A real-time fluorescence quantitative and virus titer technology, which is applied in the field of kits and real-time fluorescent quantitative PCR primers, can solve the problems of not reflecting the real infection activity of the virus, not being able to accurately measure the virus titer, and inaccurate virus titer detection results, etc. Achieve the effect of real infectious vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Design of specific primers
[0040] According to the published viral sequences, the present invention selects the envelope region of the lentivirus to design primers; the designed primers are preliminarily screened to obtain a set of primer pairs with high versatility, good sensitivity and specificity, Its nucleotide sequence is as follows:
[0041] ENV-F:CGCAGTTAATCCTGGCCTGTTA(SEQ.ID:NO.1);
[0042] ENV-R: ATCCTTTGATGCACACAATAGAGG (SEQ. ID: NO. 2).
[0043] Using the lentiviral shuttle plasmid as a template, the amplified sequence is:
[0044] cgcagttaatcctggcctgttagaaacatcagaaggctgtagacaaatactgggacagctacaaccatcccttcagacaggatcagaagaacttagatcattataataatacagtagcaaccctctattgtgtgtgcatcaaaggat(SEQ.ID:NO.3);
[0045] The length of the amplified fragment was 144 bp.
[0046] Internal reference BMP2 (Homo sapiens bone morphogenetic protein 2, bone morphogenetic protein 2) primers:
[0047] BMP2-F: TAGGGTAGACAGAGCCAAGG (SEQ. ID: NO.4);
[0048] BMP2-R: AGCACAGGA...
Embodiment 2
[0052] Example 2 Preparation of standard substance and real-time quantitative PCR (qPCR) detection
[0053] 1. Preparation of standards
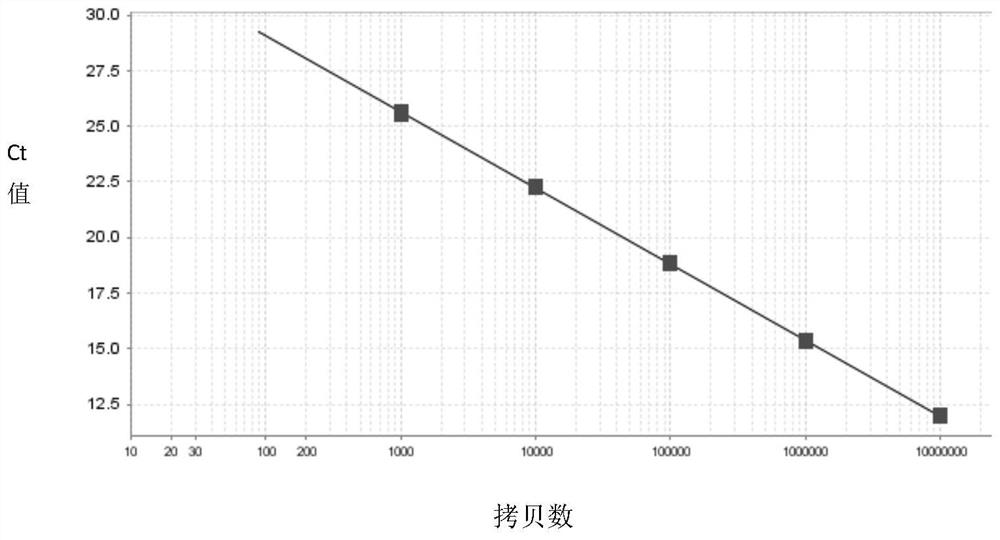
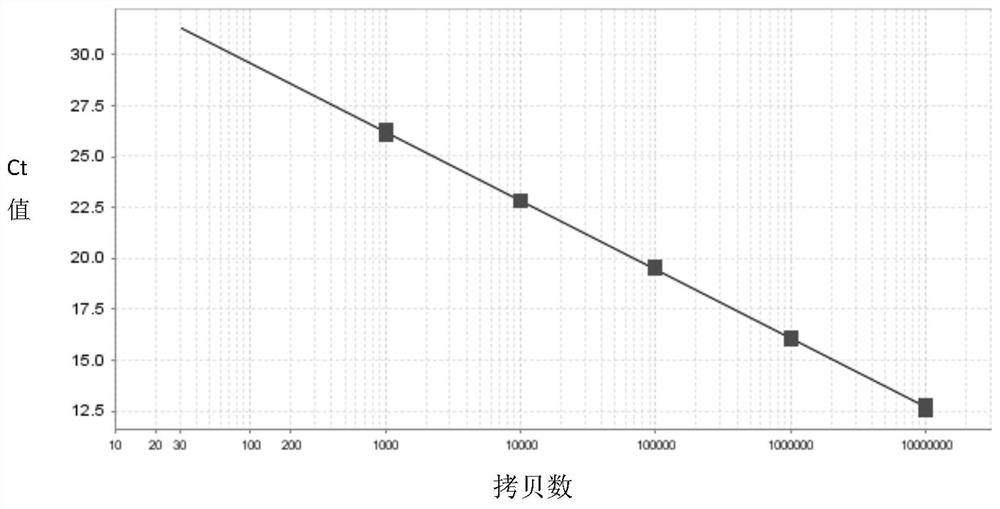
[0054] Using the ENV-F, ENV-R, BMP2-F, BMP2-R primers of Example 1, the target fragment was amplified, and the amplified product was connected to pMD TM 18-T vector. The constructed vector was double digested by AhdI and NdeI, and the digested product was recovered and its concentration and purity were determined by NanoDrop. For the samples that meet the purity, the number of molecules is calculated according to the molecular weight of the fragments and Avogadro's constant, which are used as the standard for virus quantification, which are the ENV standard and the internal reference BMP2 standard.
[0055] The specific calculation formula is: sample copy number per 1uL fragment = A × N × 10 -9 / M. where A is the DNA concentration measured in ng / uL; N is Avogadro’s constant (6.02×10 23 / mol); M is the molecular weight of the fragment.
...
Embodiment 3
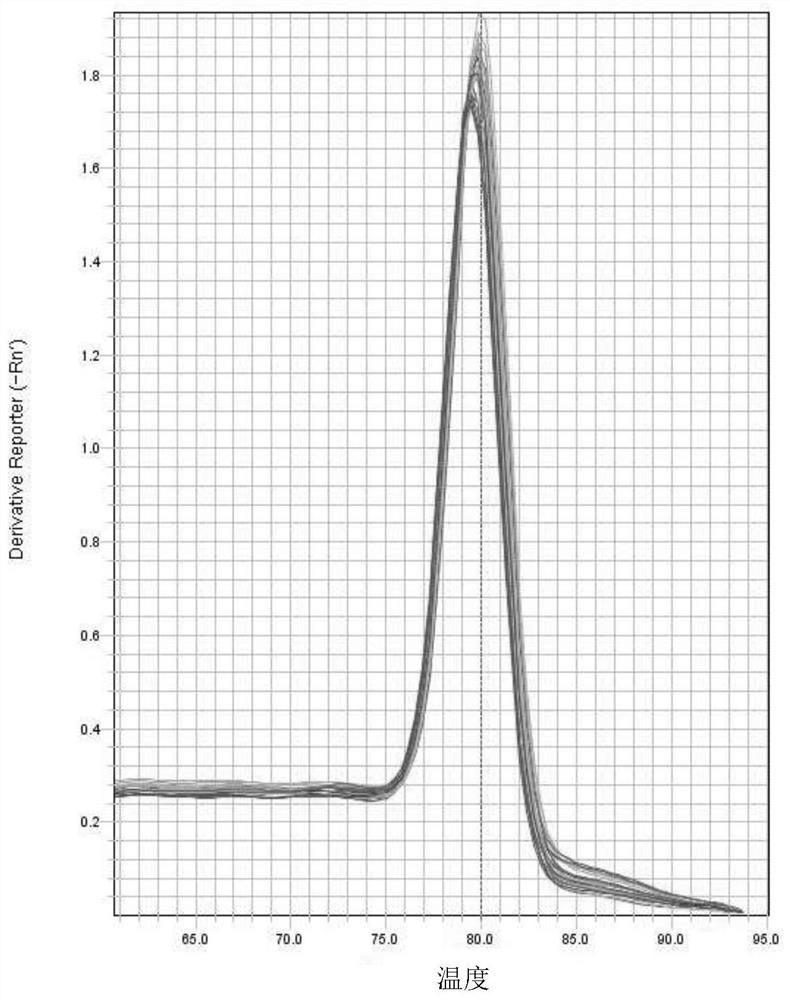
[0068] Example 3 Real-time fluorescent quantitative PCR of genomic DNA of target cells
[0069] (1) Virus-transduced 293T cells:
[0070] Take 3 x 10 per hole 5 293T cells were inoculated into 6-well plates, and the cells in one well were taken for trypsin digestion and counted before virus transduction the next day, and the counted results were saved and recorded as B. Use 293T complete medium to dilute the virus product by 100 times, add 50uL of the diluted virus solution to one well of a 6-well plate, and add 10uL, 0.5mg / mL polybrene to promote transduction, keep one Cells without any virus transduction in the well were used as a negative control and were placed in a 37°C incubator for further culture.
[0071] (2) Extraction of cellular genomic DNA:
[0072] 48 hours after viral transduction, cells were collected, and target cell genomic DNA was extracted with a blood tissue cell genome extraction kit (Tiangen, DP304).
[0073] (3) quantitative PCR analysis:
[0074] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com