Electrochemical sensor based on chiral covalent organic framework compound
A covalent organic framework and sensor technology, applied in the field of electrochemical detection, can solve problems such as design, synthesis, functionalization difficulties, and limited analysis and application, and achieve the effects of excellent capacitance performance, obvious enrichment effect, and good conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 2.5 mg of the four materials of COF-CTpPa-2, COF-TpPa-1, COF-TpPa-2 and COF-TpBD respectively, dissolve them in 5.0 mL of ethanol-Nafion solution, and continue ultrasonication for 3 hours until the materials are evenly distributed in, And refrigerated storage at 4 °C; use 3 μm and 0.05 μm alumina powder to grind and polish the electrode, and then in turn, the electrode is ultrasonicated in nitric acid (concentrated nitric acid:water, 1:3, V:V) for 30 s, and in ethanol for 1 min. Sonicate in water for 1 min, blow dry with nitrogen; drop 5 μL of the four material solutions on the electrodes, and then dry them at room temperature, and the electrochemical sensors of the four materials are prepared.
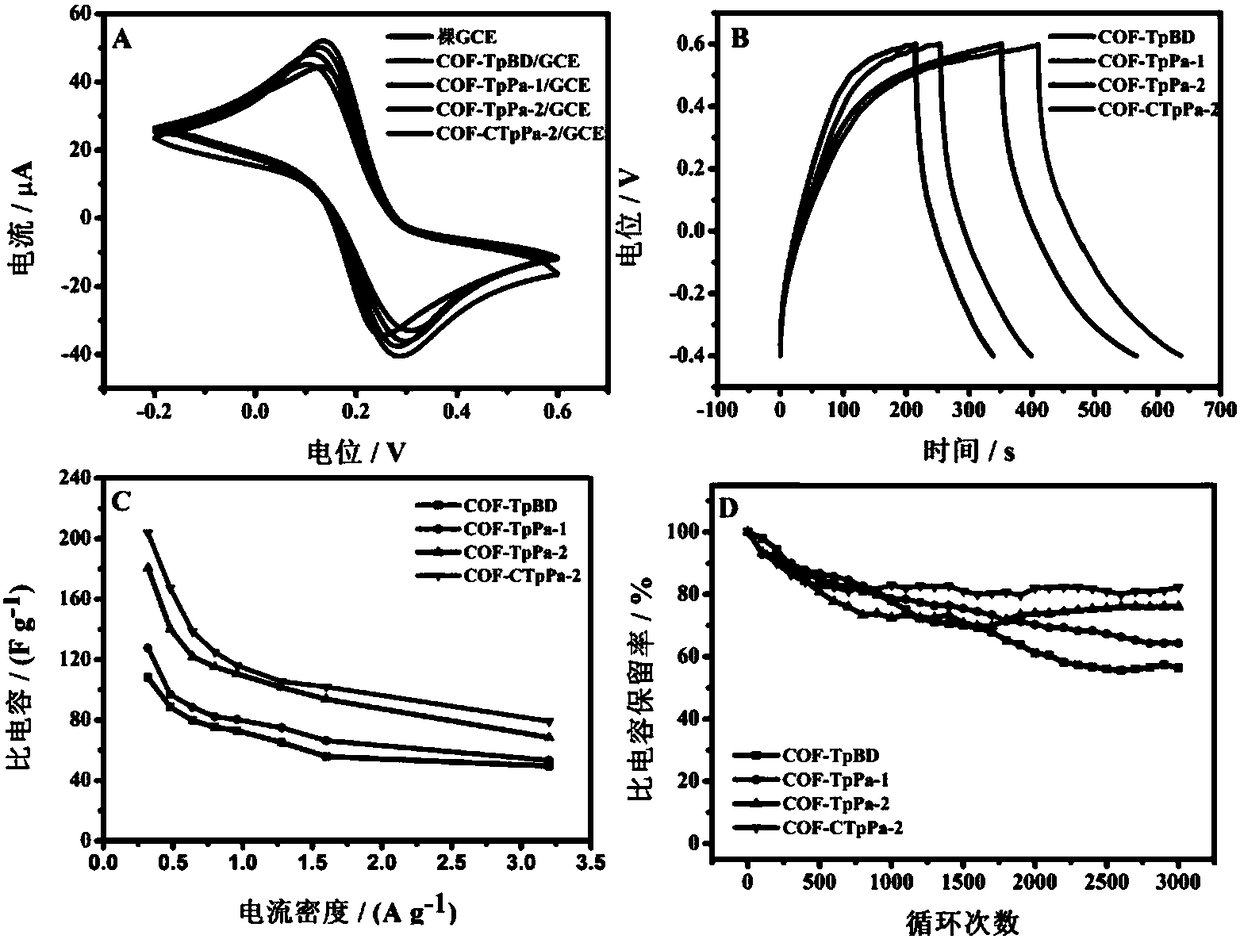
[0037] Such as figure 1 Shown are the effects of electrochemical sensors modified by four different COF materials: COF-CTpPa-2, COF-TpPa-1, COF-TpPa-2 and COF-TpBD.
[0038] The prepared electrical sensor was placed in 1 mM potassium ferricyanide solution, the scanning r...
Embodiment 2
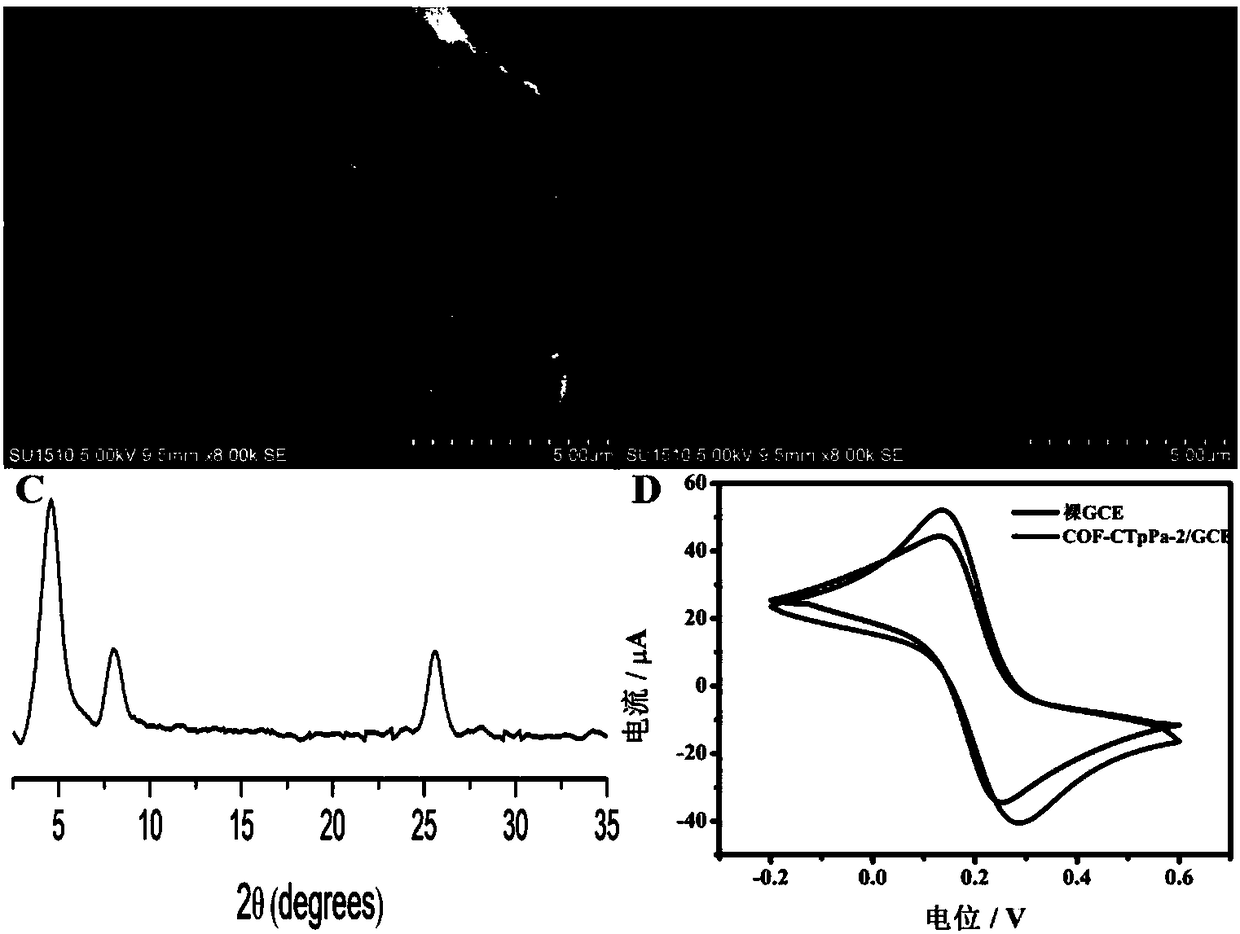
[0040] Such as figure 2 Shown, is the principle verification of the present invention.
[0041] The COF-CTpPa-2 electrochemical sensor prepared in Example 1 was characterized by SEM and XRD diffraction, figure 2 A and figure 2 B are the SEM images of the COF-CTpPa-2 modified electrode and the electrode, respectively. It can be seen from the figure that the surface of the glassy carbon electrode modified by COF-CTpPa-2 is wrinkled and distributed with small holes of uniform pore size. Wrinkles and small holes increase the effective area of the electrode surface, which is beneficial to the further enrichment of the analyte. figure 2 C is the XRD diffraction pattern of COF-CTpPa-2. It can be seen from the figure that COF-CTpPa-2 has diffraction peaks at 4.7°, 8.1° and 25.2°, which is consistent with the literature reports, indicating that the synthesized is COF - CTpPa-2 compound.
[0042] The prepared electric sensor was placed in 1mM potassium ferricyanide solution, t...
Embodiment 3
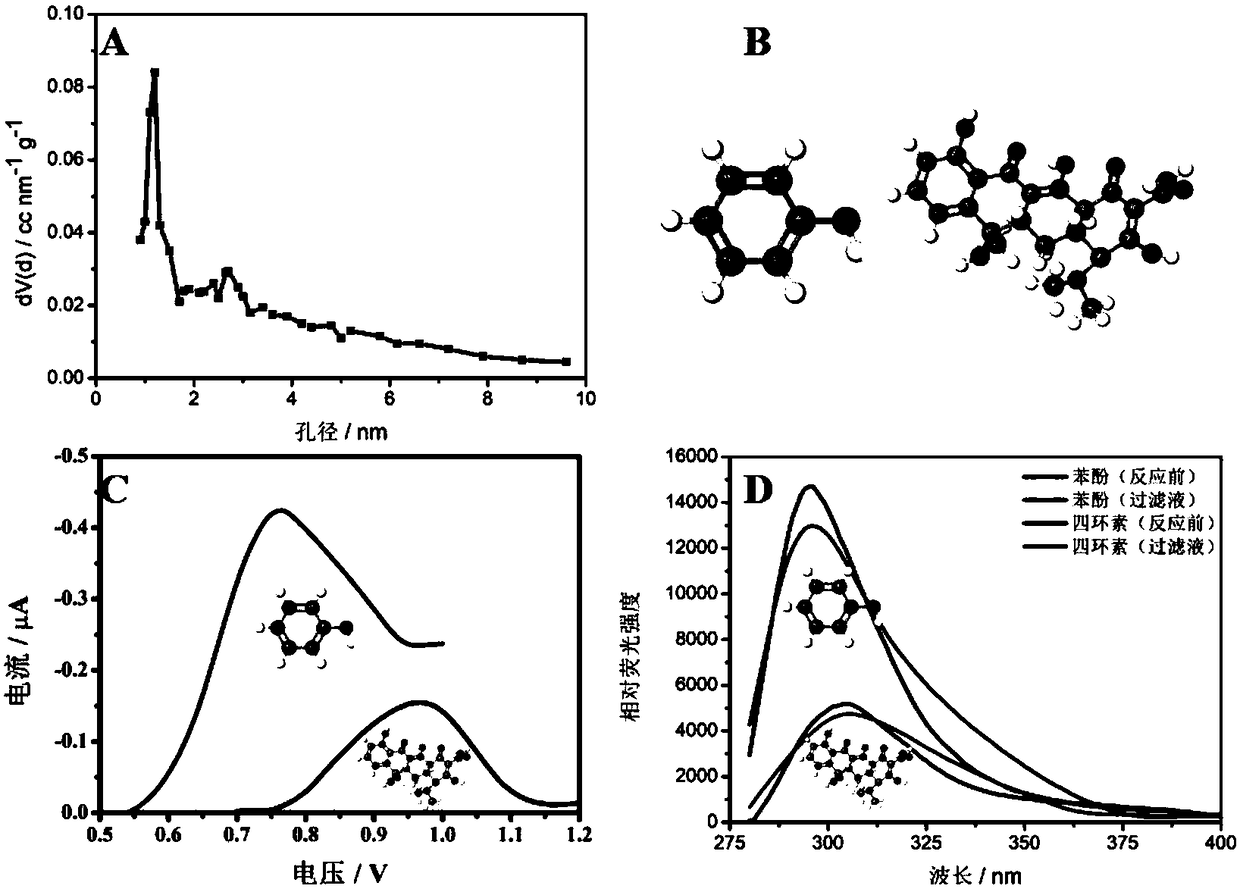
[0044] Such as image 3 Shown is the application of COF-CTpPa-2 electrochemical sensor to electroactive substances with π-conjugated structure and different molecular diameters.
[0045] Depend on image 3 A It is known that the pore size of COF-CTpPa-2 is about Draw phenol and tetracycline through online painting software, and get the molecular diameters of phenol and tetracycline through testing tools as with
[0046] The prepared COF-CTpPa-2 electrochemical sensor was placed in an electrolyte solution containing 50 μM phenol and tetracycline respectively, connected to a power supply, and scanned by differential pulse voltammetry with a scanning potential of -0.5V to 1.2V.
[0047] image 3 A is the pore diameter of COF-CTpPa-2, image 3 B is the molecular diameter of phenol and tetracycline, it can be seen that the pore diameter of CTpPa-2 is about The molecular diameters of phenol and tetracycline are with image 3 C is the electrochemical response diagram ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com