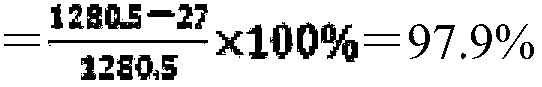Method using sea water or brine to produce ammonium magnesium phosphate
A technology of magnesium ammonium phosphate and salt brine, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., to achieve the effect of high recovery rate and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Use sea water to produce ammonium magnesium phosphate by alkali method
[0051] A. Inducer: monoammonium phosphate + disodium hydrogen phosphate
[0052] B. Preparation of alkaline diluent
[0053] First dissolve 3g of anionic polyacrylamide in 800ml of deionized water, stir for more than 2 hours to fully dissolve; then add 135.0g of solid sodium hydroxide and 10.0g of powdered activated carbon, and dilute to a 1000ml volumetric flask with deionized water .
[0054] C. The reaction produces ammonium magnesium phosphate
[0055] Add 6 grams of monoammonium phosphate and 4 grams of disodium hydrogen phosphate to 1 kilogram of sea water, stir for 30 minutes, and fully dissolve. Then add 29ml of diluent, continue to stir, react for more than 30 minutes, the pH value of the solution stabilizes at 8.1. Let stand and settle for 2 hours.
[0056] Skim the supernatant, precipitate and filter, and dry at 50°C to obtain 9.45 g of white powder.
[0057] Through X-ray diffractometer analysis...
Embodiment 2-9
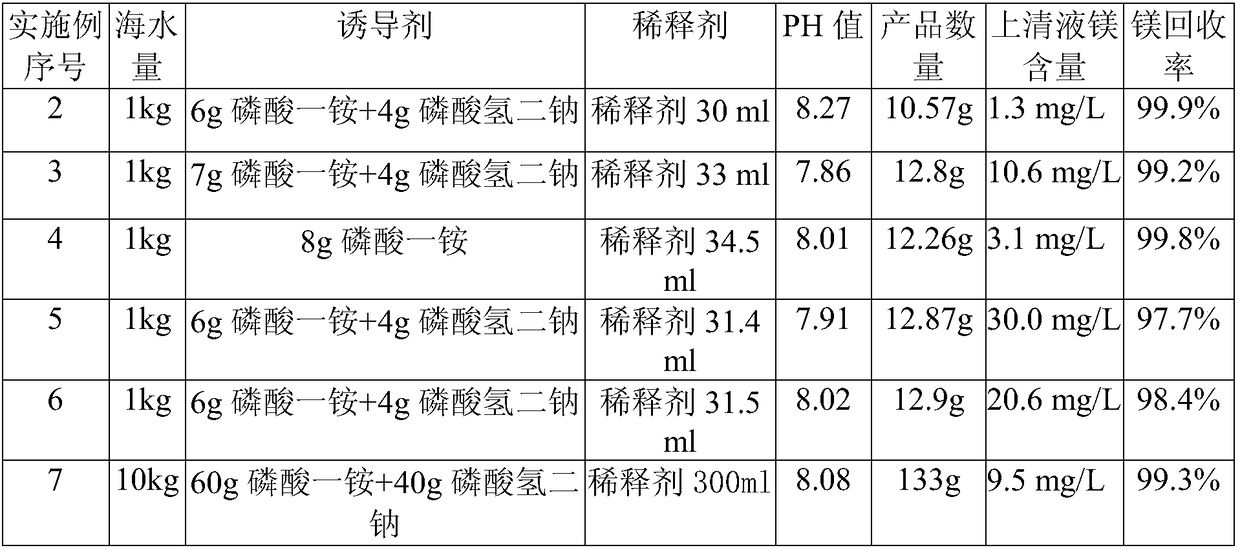
[0063] The basic situation is the same as in Example 1, except that the amount of seawater, the amount and combination of inducers, and the amount of diluents are adjusted to prepare magnesium ammonium phosphate. See Table 2 for details.
Embodiment 2 to 9
[0065]
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com