Preparation method of lipoic acid impurity H
A lipoic acid and impurity technology, which is applied in the field of preparation of lipoic acid impurity H, can solve problems such as the large difference between the prescription process and product quality control level, unfavorable product quality control supervision, unstable high-temperature sterilization of lipoic acid injection, etc. To meet the needs of quality control, the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Confirmation of the lipoic acid impurity H of embodiment 1 present invention
[0027] ①Refer to the known impurities recorded in the lipoic acid API in the European Pharmacopoeia (EP) as follows:
[0028]
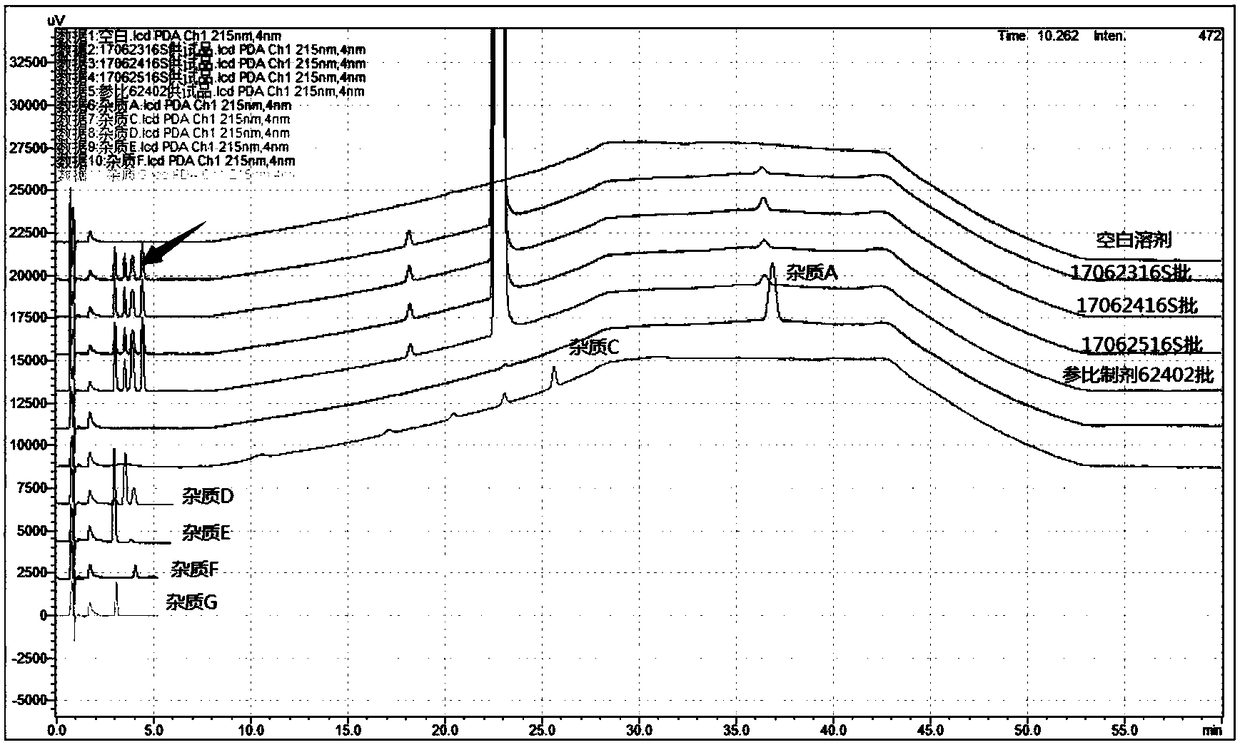
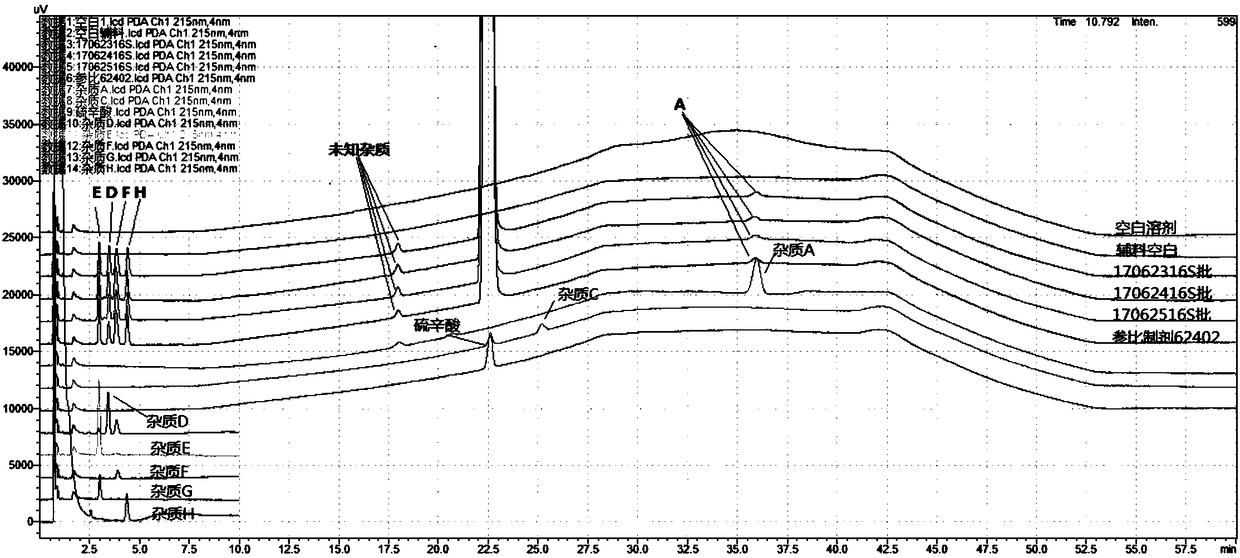
[0029] ② Through the analysis and location research of the degraded impurities in the preparation process of lipoic acid raw materials, the impurities C~G that may be produced in the finished lipoic acid injection were sorted out, and the impurities C were degraded impurities in the preparation process of lipoic acid raw materials, and the impurities D~G It is an oxidized impurity in the finished product of lipoic acid raw material medicine. The strong degradation test analyzes the source of the impurity including photodegradation, oxidative degradation and high temperature degradation. For the specific location map, see figure 1 . At the same time, it was also found that another unknown impurity whose source and location cannot be known, as indicated by the red ...
Embodiment 2
[0030] The preparation of embodiment 2 lipoic acid impurity H of the present invention
[0031] Preparation of impurity H reaction equation:
[0032]
[0033] Add 10.0g of lipoic acid and 50ml of dried chloroform into a 500ml three-necked flask. Stir to dissolve, add 9.43g of carbonyldiimidazole, and stir at 20-30°C for 30min. After the temperature of the system was lowered to 0-4°C, 17.85 g of ethylenediamine was dissolved in 90 ml of dried chloroform, and then added dropwise to the reaction system. After the feeding is completed, the temperature is raised to 20-30° C. and the temperature is controlled for 8-10 hours until the reaction of the intermediate product is complete.
[0034] After the reaction was complete, the reaction solution was washed three times with 50 ml of 1 mol / L sodium hydroxide solution. After adding 50ml of purified water, add 0.05mol / L hydrochloric acid to adjust the pH to 2.5. Stir for 5 minutes, filter to remove incompatible substances, and the...
Embodiment 3
[0038] Embodiment 3 Preparation process parameter selection test of lipoic acid impurity H of the present invention
[0039] Comparative test one, the amount of raw material input is shown in the table below, and all the other operating steps are completely the same as embodiment 2:
[0040]
[0041] Contrast test 2, after the reaction is complete, whether to adjust the pH value and the pH value range during the washing process are as follows, and the rest of the operation steps and raw material dosage are the same as the above process:
[0042]
2mol / L hydrochloric acid to adjust pH
3mol / L sodium hydroxide to adjust pH
Finished product purity (%)
Example 1
2.5
12
95
Example 3
2
12
95
Example 4
3
11.5
92
Example 5
3
12
93
Comparative example 6
——
——
23
Comparative example 7
——
11
45
Comparative example 8
2.5
——
39
Comparative example 9
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com