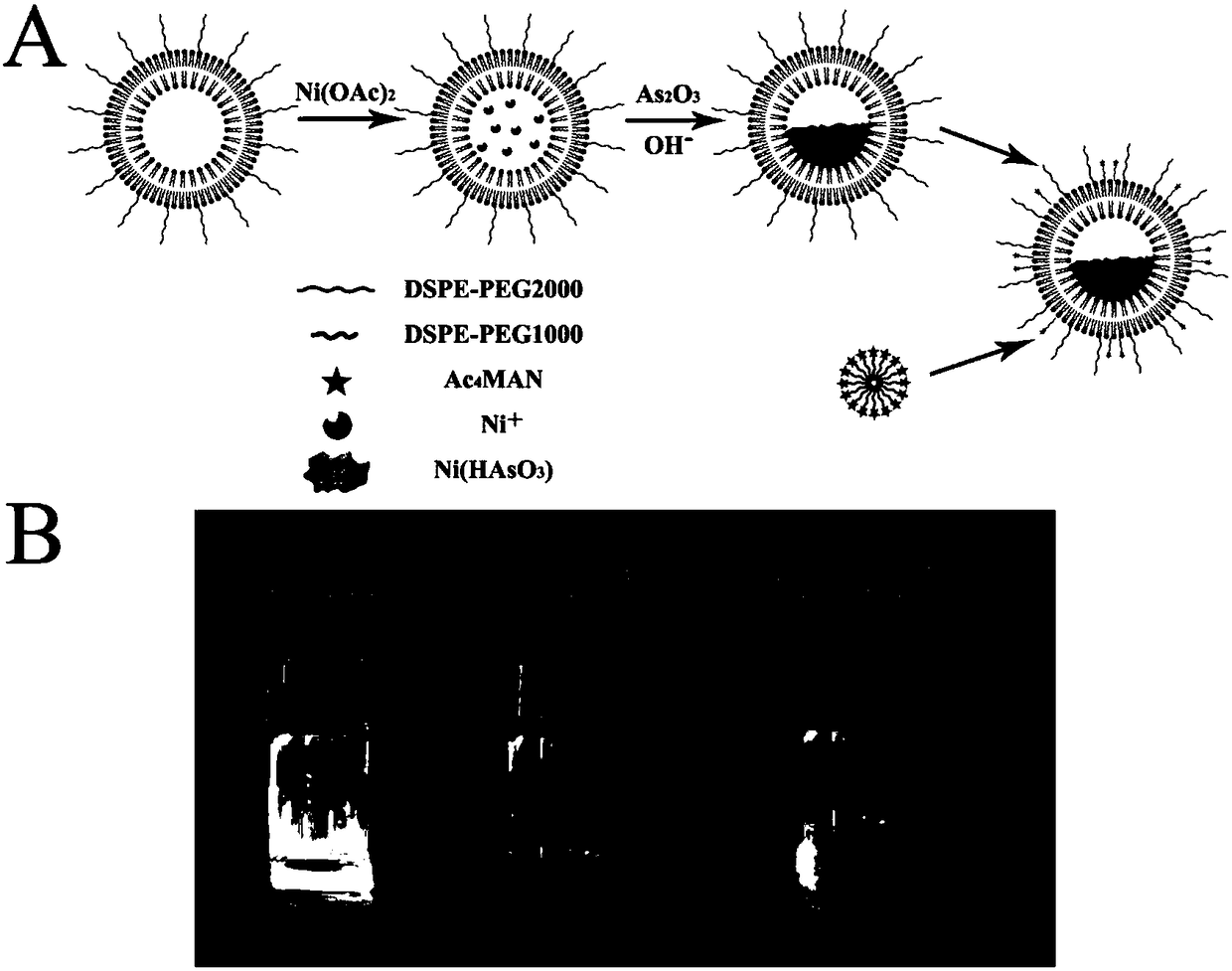
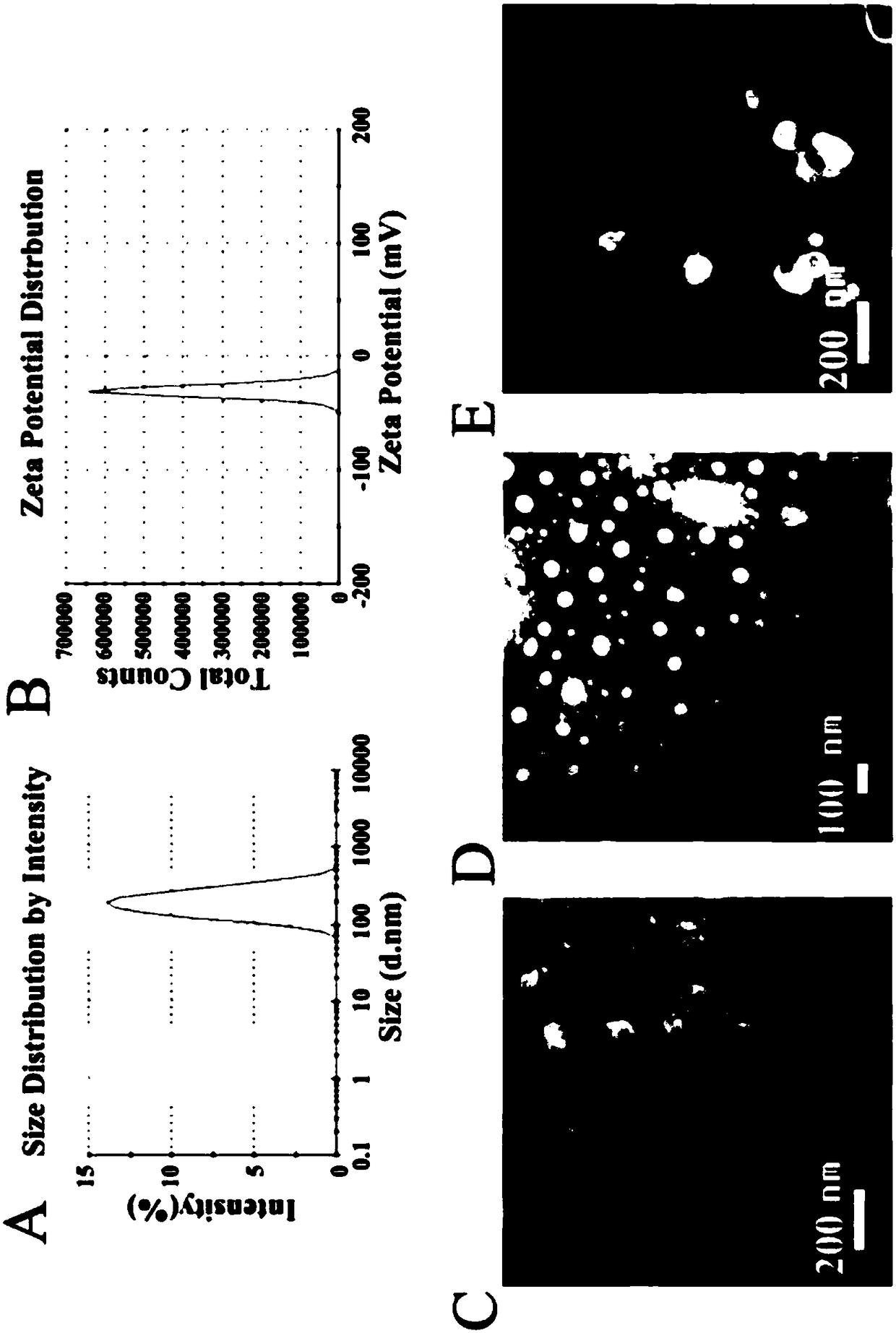
Acetylation sugar ester-polyethylene glycol-phosphatidyl ethanolamine conjugate and preparation method and application thereof
A technology of phosphatidylethanolamine conjugates and distearoylphosphatidylethanolamine, applied in the field of medicine, can solve the problems that hinder the application and effect of solid tumor treatment, and the mechanism has not been fully understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
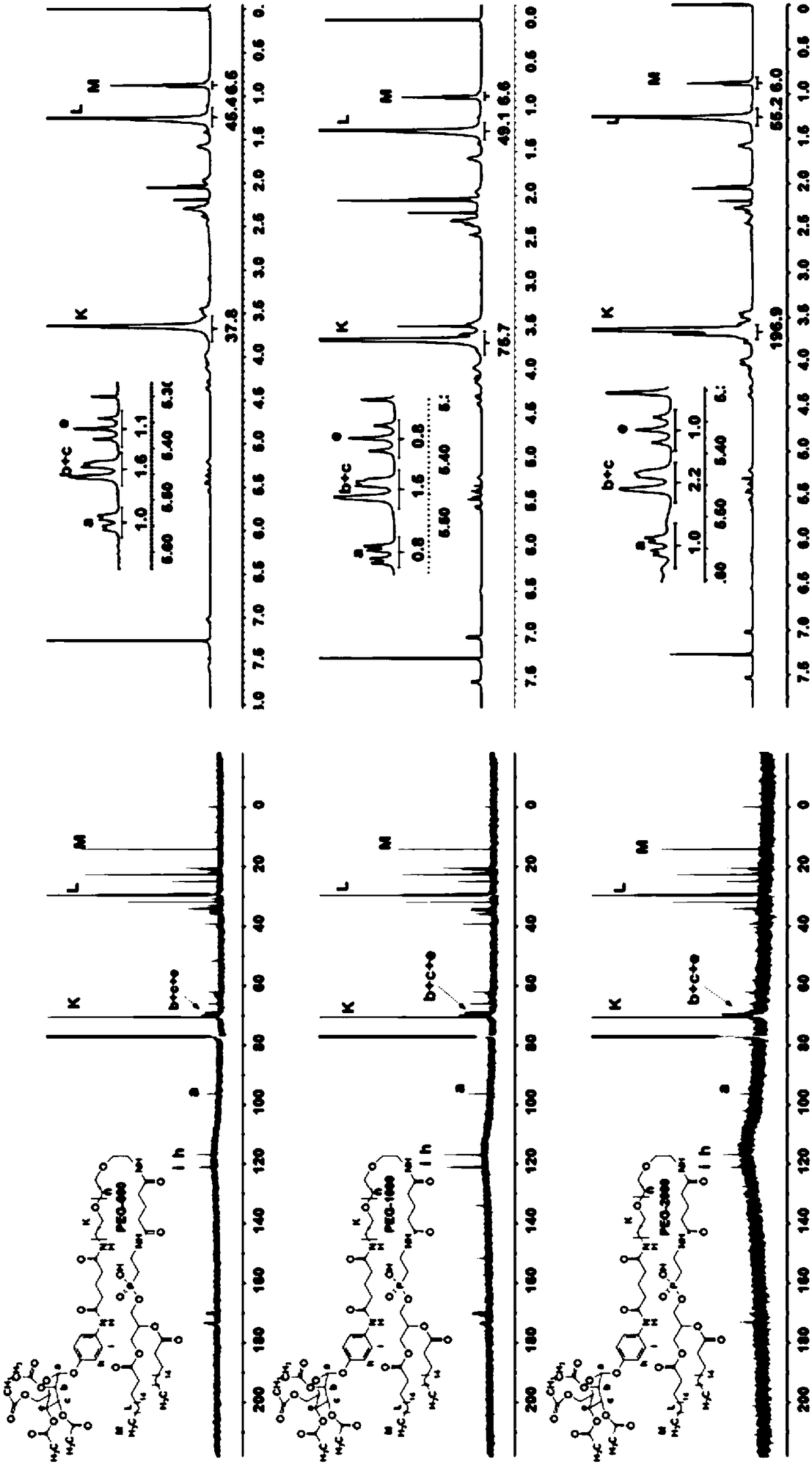
[0068] Example 1 Synthesis of targeting molecule diisoamylphosphoethanolamine-polyethylene glycol-1000-p-p-carboxyphenyl-α-D-acetylmannosamine (DSPE-PEG-1000-Ac4MAN)
[0069]
[0070] Azide-polyethylene glycol-carboxy 1000 (1.3 g) was dissolved in tetrahydrofuran (20 mL), then Pd / c (0.066 g) and acetic acid (1 mL) were added, and hydrogen gas was introduced at room temperature to react overnight. After the crude product was filtered, it was rotary evaporated to dryness, then dissolved in dichloromethane (20 mL), triethylamine (TEA, 0.65 mL) and Ac 4 MAN (1.5g) was reacted overnight at room temperature. Go through the column to get PEG1000-Ac 4MAN (1.18 g), 62% yield. Dissolve PEG 1000-Ac4MAN (1.18g) in dichloromethane, add NHS (0.2g) and EDCI (0.45g) in sequence, react overnight at room temperature, and pass through the column to obtain succinimide polyethylene glycol acetyl mannoside (1.0 g), 78% yield. Dissolve succinimide polyethylene glycol acetyl mannoside (0.95g) ...
Embodiment 2
[0072] Example 2 Targeting molecule Diisoamylphosphoethanolamine-polyethylene glycol-600-p-p-carboxyphenyl-α-D-acetylmannosamine (DSPE-PEG-600-Ac 4 MAN) synthesis
[0073]
[0074] Azide-polyethylene glycol-carboxy 600 (1.3 g) was dissolved in tetrahydrofuran (20 mL), then Pd / c (0.078 g) and acetic acid (1 mL) were added, hydrogen gas was introduced at room temperature, and the reaction was carried out overnight. After the crude product was filtered, it was rotary evaporated to dryness, then dissolved in dichloromethane (20 mL), added triethylamine (TEA, 0.79 mL) and Ac4MAN (1.7 g), and reacted overnight at room temperature. After passing through the column, PEG600-Ac4MAN (1.08 g) was obtained with a yield of 62%. Dissolve PEG600-Ac4MAN (0.95g) in dichloromethane (20mL), add NHS (0.3g) and EDCI (0.51g) in turn, react at room temperature overnight, and pass through the column to obtain succinimide polyethylene glycol acetylene Mannoside (0.82 g), 80% yield. Add succinimid...
Embodiment 3
[0076] Example 3 Targeting molecule Diisoamylphosphoethanolamine-polyethylene glycol-2000-p-p-carboxyphenyl-α-D-acetylmannosamine (DSPE-PEG-2000-Ac 4 MAN) synthesis
[0077]
[0078] Azide-polyethylene glycol-carboxy 2000 (1.3 g) was dissolved in tetrahydrofuran (20 mL), then Pd / c (0.052 g) and acetic acid (1 mL) were added, and hydrogen gas was introduced at room temperature to react overnight. After the crude product was filtered, it was rotary evaporated to dryness, then dissolved in dichloromethane (20 mL), added triethylamine (TEA, 0.54 mL) and Ac4MAN (1.34 g), and reacted overnight at room temperature. Through the column, PEG2000-Ac4MAN (1.15 g) was obtained with a yield of 63%. Dissolve PEG 2000-Ac4MAN (0.95g) in dichloromethane (20mL), add NHS (0.09g) and EDCI (0.19g) in turn, react overnight at room temperature, and pass through the column to obtain succinimide polyethylene glycol Acetylmannoside (0.87g), 82% yield. Add TEA (0.15 mL) and DSPE (0.3 g) dissolved i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com