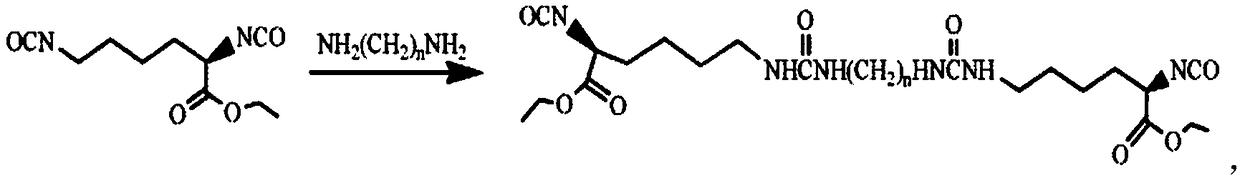
Biodegradable high-strength polyether ester type polyurethane urea foam and preparation method thereof
A technology of polyurethane urea and polyether ester is applied in the field of preparation of fully synthetic biodegradable hemostatic foam, which can solve the problems of difficult adjustment of the ratio of soft and hard segments, difficult control of the content of hydrophilic segments, unsatisfactory hemostatic effect, and the like. The effect of compression hemostasis, excellent water absorption and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of diisocyanate chain extender containing ureido structure:
[0064] Preparation of LBL: Under dry nitrogen protection and mechanical stirring, add 1,4-butanediamine dropwise to L-lysine diisocyanate (-NCO:-NH 2 =8:1, molar ratio), after reacting at room temperature for 2h, add four times the volume of n-hexane to the reaction product, after stirring evenly, obtain a white solid by suction filtration, wash with n-hexane repeatedly until the filtrate IR detects that there is no -NCO absorption Peak (2270cm -1 ), vacuum-dried to constant weight to obtain white powder LBL.
[0065] Preparation of LHL: Add 1,6-hexanediamine dropwise to L-lysine diisocyanate (-NCO:-NH 2 =10:1, molar ratio), after reacting at room temperature for 2h, add four times the volume of n-hexane to the reaction product, after stirring evenly, obtain a white solid by suction filtration, wash with n-hexane repeatedly until the filtrate IR detects that there is no -NCO absorption Peak (22...
Embodiment 2
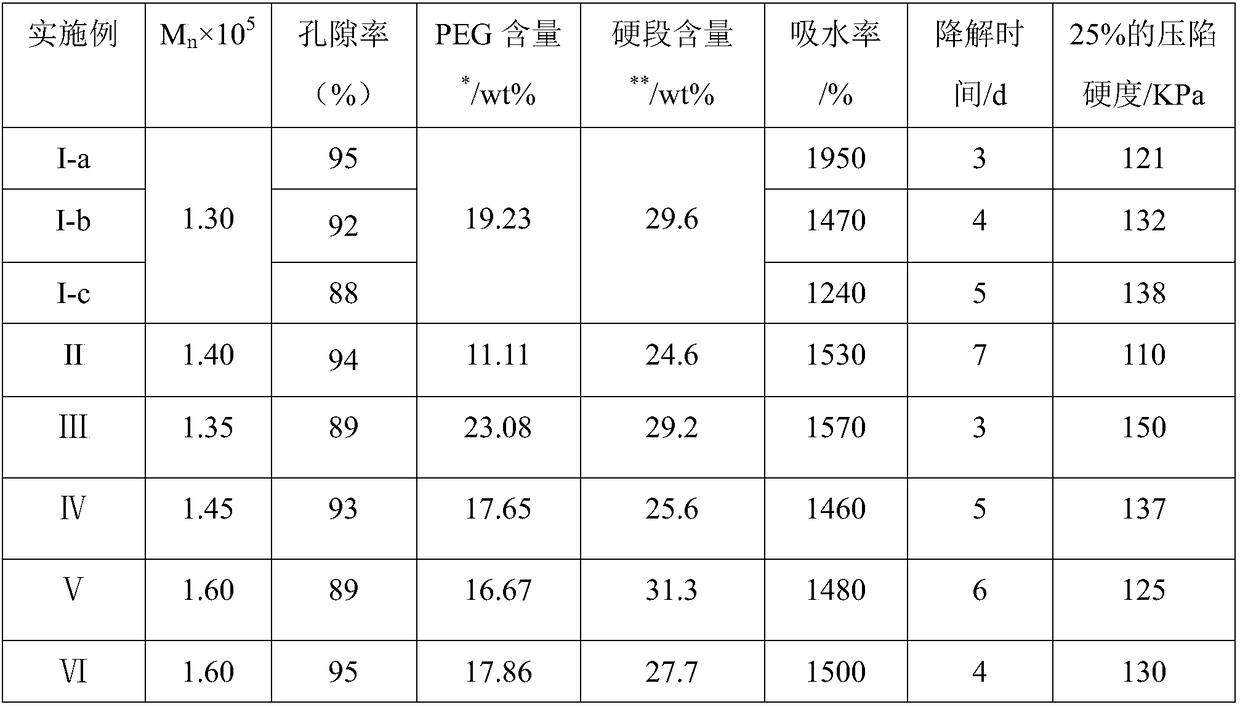
[0067] Take 10.0g (7.7mmol) PPDO-PEG-PPDO (M n =1300, the PEG content is 19.23wt%) was added to N,N-dimethylformamide (DMF) to dissolve (0.5g / mL), the reaction system was cooled to 85°C, and the DMF solution of LBL (8.0mmol) was added dropwise ( 1.0g / mL), after the dropwise addition, keep the temperature and continue to react for 4h, lower to room temperature, then add DMF to make a solution with a concentration of about 10g / 100mL, 7 times the volume of glacial ether settles, and the obtained solid is vacuum-dried at 35°C to obtain polyether Ester polyurethane urea;
[0068] The polyurethane urea was dissolved in dioxane at concentrations of 4.5g / 100mL, 5.0g / 100mL, and 5.5g / 100mL, pre-frozen at -15.5°C for 4 hours, and then vacuum-frozen at -50°C to obtain a foam. Denote as samples I-a, I-b, and I-c, respectively.
Embodiment 3
[0070] Take 8.0g (4.4mmol) PPDO-PEG-PPDO (M n =1800, the PEG content is 11.11wt%) was added to N,N-dimethylformamide (DMF) to dissolve (0.5g / mL), the reaction system was cooled to 80°C, and the DMF solution of LHL (4.6mmol) was added dropwise ( 1.0g / mL), after the dropwise addition, keep the temperature and continue to react for 5h, lower to room temperature, then add DMF to make a solution with a concentration of about 9g / 100mL, 8 times the volume of glacial ether settles, and the obtained solid is vacuum-dried at 35°C to obtain polyether Ester polyurethane urea;
[0071] The polyurethane urea was dissolved in dioxane at a concentration of 5.0 g / 100 mL, and freeze-dried to obtain a foam. Denoted as sample II.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com