Method for one-step synthesis of non-steroidal anti-inflammatory drug diflunisal
A technology of medicine fluorobenzenesalicylic acid and fluorobenzenesalicylic acid, which is applied in the field of catalytic organic synthesis, can solve the problems of environmental hazard, air, humidity sensitivity, etc., and achieves a short reaction time, few by-products, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
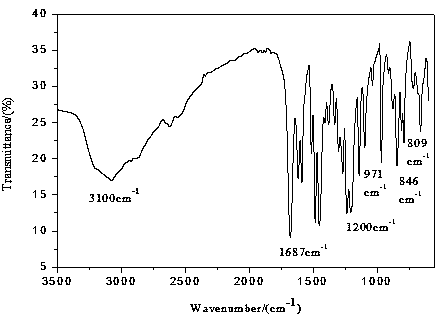
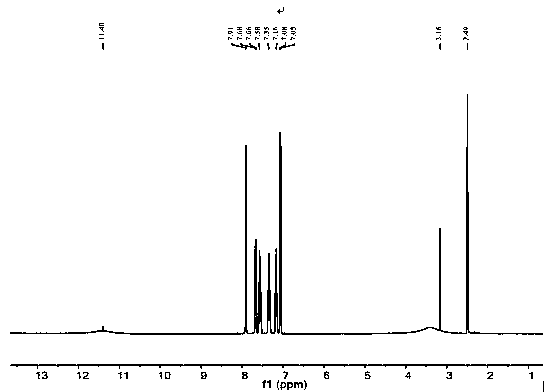
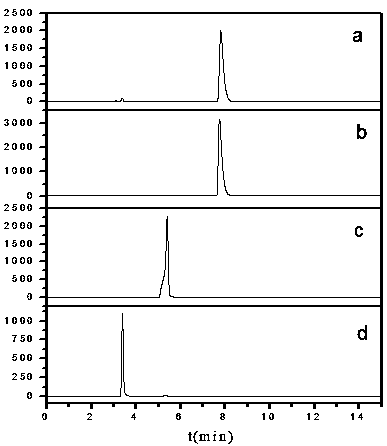
Image
Examples
Embodiment 1
[0027] Embodiment 1: Ultrasonic Synthesis of Fluorben Salicylic Acid
[0028] Mix 120ml of distilled water and 120ml of N,N-dimethylformamide into the ultrasonic reactor and ultrasonically mix to form solution A, then add 53.2mg of PdCl 2 (0.03mmol) ultrasonically disperse in a cold water bath, add 7.2g 2,4-difluorophenylboronic acid (45mmol), 6.51g 5-bromosalicylic acid (30 mmol) ultrasonically dissolve and disperse in a cold water bath, and finally add 3.36 g K 2 CO 3 (60mmol) ultrasonically dissolved and dispersed, and reacted in an ultrasonic reactor with a temperature of 75°C and an ultrasonic power of 175W for 100min. The flocculent precipitate no longer disappears, then add 2000ml of distilled water to the filtrate, let it stand until the flocculent precipitate of flufenesalic acid is completely precipitated, filter to obtain a white filter cake, dissolve the filter cake with 200ml of ethyl acetate, and wash with deionized water Wash 3 times, take the upper organic ph...
Embodiment 2
[0029] Example 2 Conventional Synthesis of Fluorben Salicylic Acid
[0030]Add 120ml of distilled water and 120ml of N,N-dimethylformamide into a reactor equipped with a condensing device, stir and mix to form a mixed solution A, and then add 53.2mg of PdCl 2 (0.03mmol) ultrasonically dispersed in a cold water bath, then added 7.2 g 2,4-difluorophenylboronic acid (45 mmol), 6.51 g 5-bromosalicylic acid (30 mmol) ultrasonically dissolved and dispersed in a cold water bath, and finally 3.36 g K 2 CO 3 (60mmol) ultrasonically dissolved and dispersed, and mechanically stirred at 75°C for 100min. After the reaction is completed, filter the reacted liquid over the catalyst, add HCl with a concentration of 1mol / L to the filtrate and shake until the precipitated flocculent precipitate no longer disappears, then add 2000ml of distilled water to the filtrate, and let it stand until the fluorobenzene water The flocculent precipitate of sylic acid was completely precipitated, and the wh...
Embodiment 3
[0031] Example 3 The operation steps are the same as in Example 1, but the reaction power is changed to 200W, and the product yield of Fluorben salicylic acid is 89.46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com