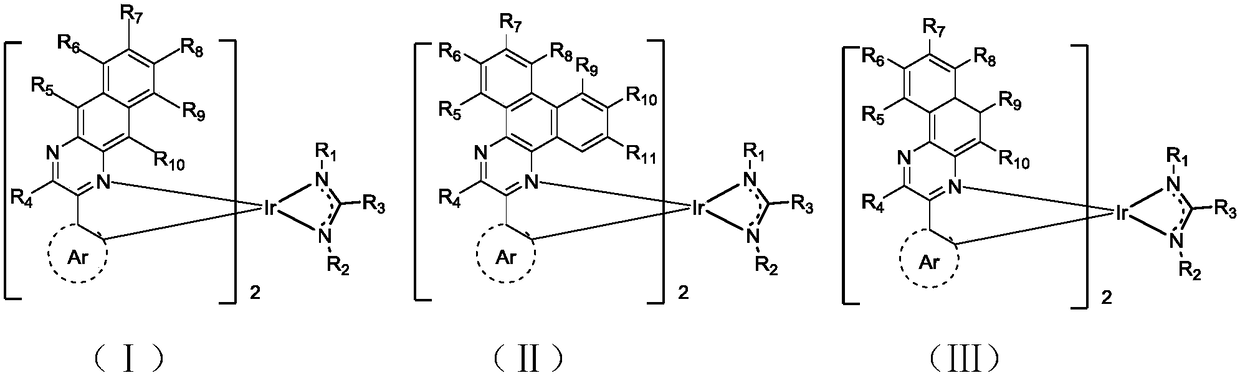
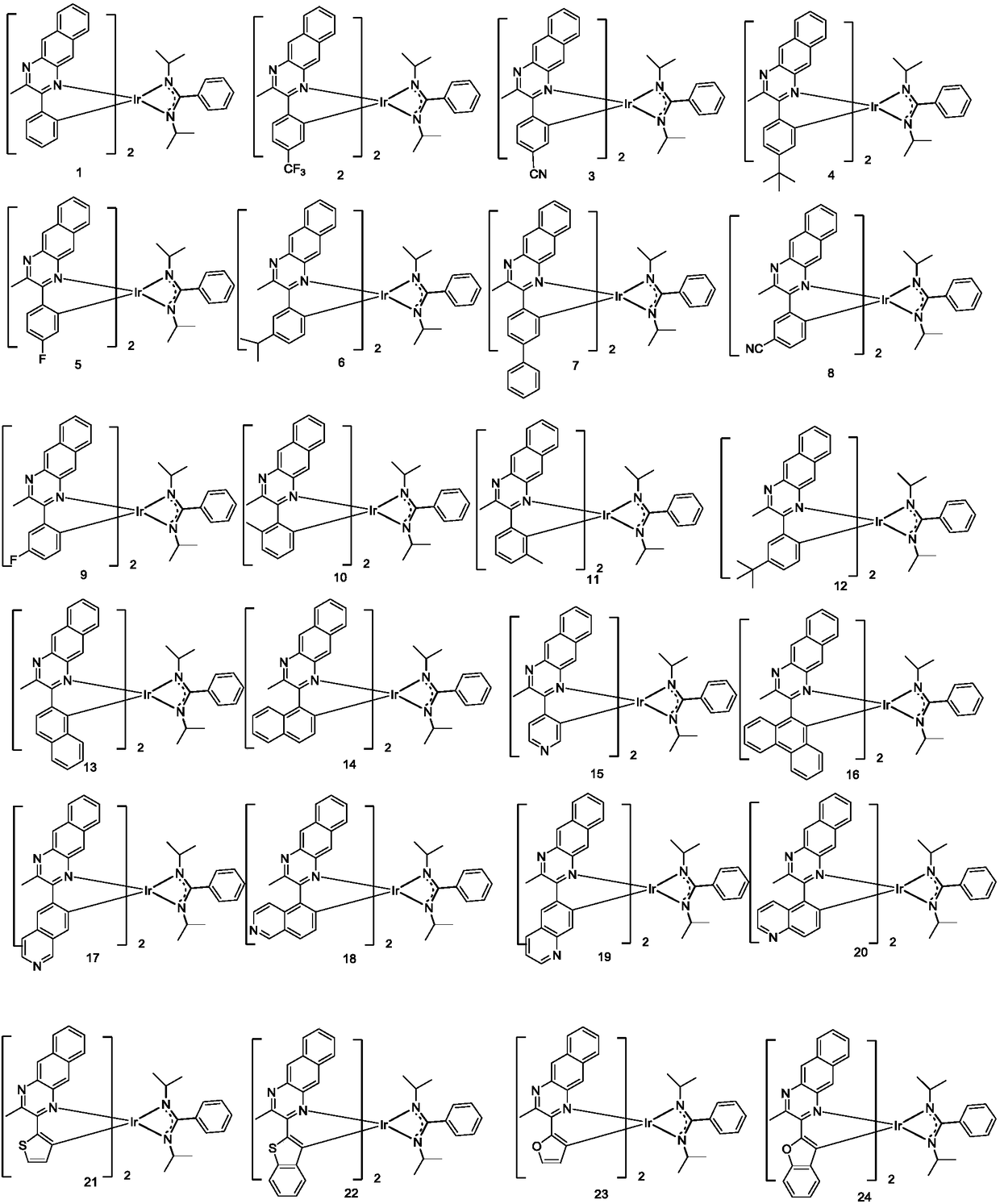
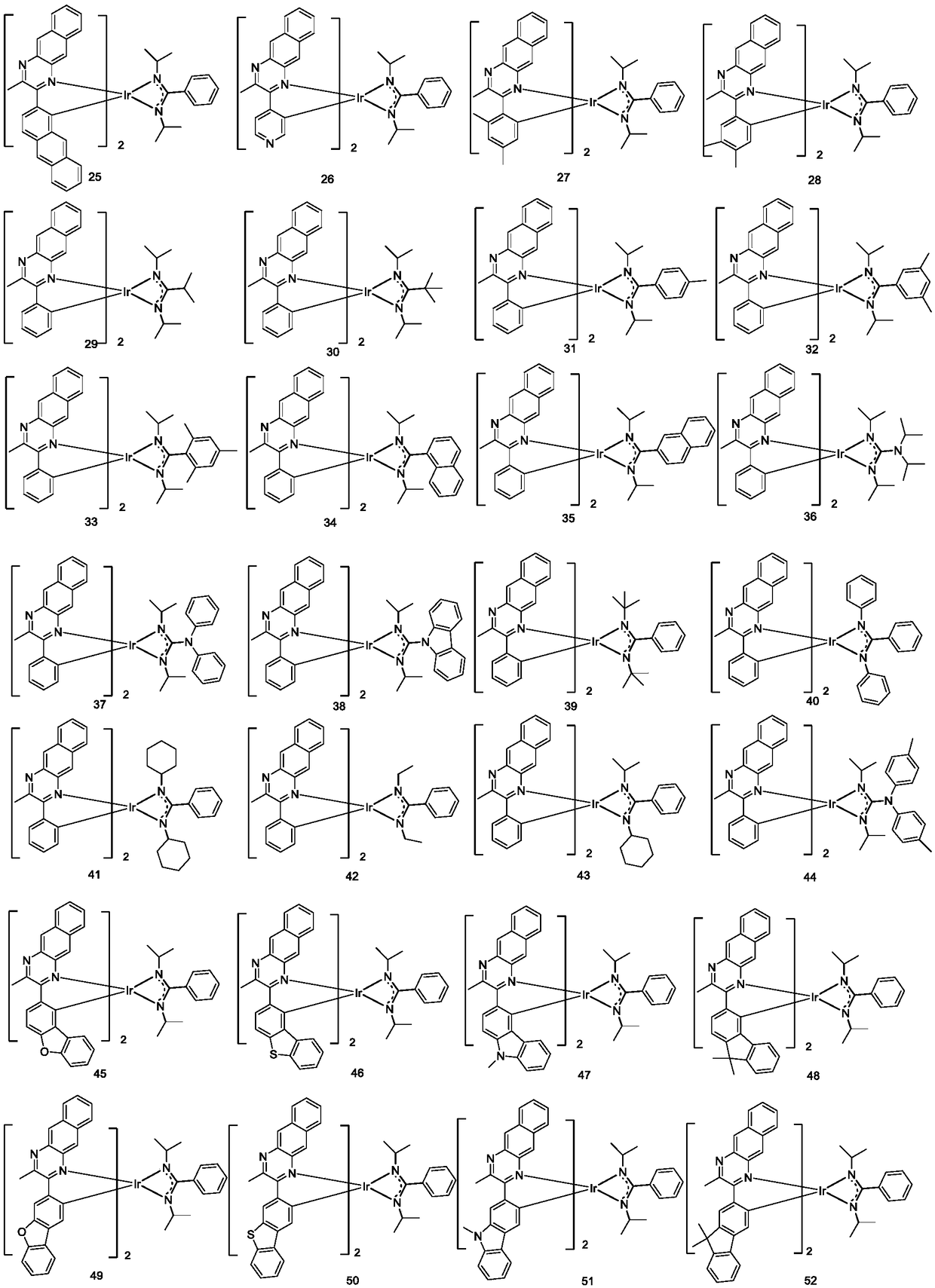
Complex and OLED (organic light emitting diode) with same
A technology of organic light-emitting devices and complexes, which is applied in the fields of light-emitting materials, organic chemistry, and electric solid-state devices, and can solve the problems of high driving voltage, low light-emitting efficiency, and poor thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: the preparation of compound 1
[0072]
[0073] Preparation of Intermediate A1
[0074] Dissolve 1.66 g (10.5 mmol) of 2,3-diaminonaphthalene and 1.4 g (10.0 mmol) of 1-phenyl-1,2-propanedione in 30 ml of absolute ethanol, and stir at reflux for 5 hours. After cooling to room temperature, the resulting solution was concentrated and separated by column chromatography, using petroleum ether / ethyl acetate=12 / 1 as the eluent, to obtain a yellow solid powder with a yield of 80%, and compound intermediate A1 was obtained.
[0075] Preparation of Intermediate B1
[0076] In a 1L three-necked flask, iridium trichloride hydrate (14.11g, 40mmol) and intermediate A1 (27.23g, 170mmol) were added, then 300mL of 2-ethoxyethanol and 100mL of water were added, and the mixture was refluxed overnight under a nitrogen atmosphere. After the reaction, the temperature was lowered to room temperature, the precipitate was filtered, washed with methanol, and dried to obtain i...
Embodiment 2
[0081] Embodiment 2: the preparation of compound 10
[0082] The b1 in Example 1 was replaced by equimolar b10, and the other steps were the same as in Example 1 to obtain the target compound 10 (5.59 g, 29%).
[0083]
[0084] Mass Spectrum m / z: 963.38 (calculated: 963.37). Theoretical element content (%)C 53 h 50 IrN 6 : C, 66.09; H, 5.23; Ir, 19.96; N, 8.72 Measured element content (%): C, 66.09; H, 5.22; Ir, 19.97; N, 8.72. The above results confirmed that the obtained product was the target product.
Embodiment 3
[0085] Embodiment 3: the preparation of compound 29
[0086] The c1 (bromobenzene) in Example 1 was converted into equimolar c29 (isopropyl bromide), and the other steps were the same as in Example 1 to obtain the target compound 29 (5.23 g, 29%).
[0087]
[0088] Mass Spectrum m / z: 901.37 (calculated: 901.36). Theoretical element content (%)C 48 h 48 IrN 6 : C, 63.98; H, 5.37; Ir, 21.33; N, 9.33 Measured element content (%): C, 63.99; H, 5.36; Ir, 21.33; N, 9.33. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com