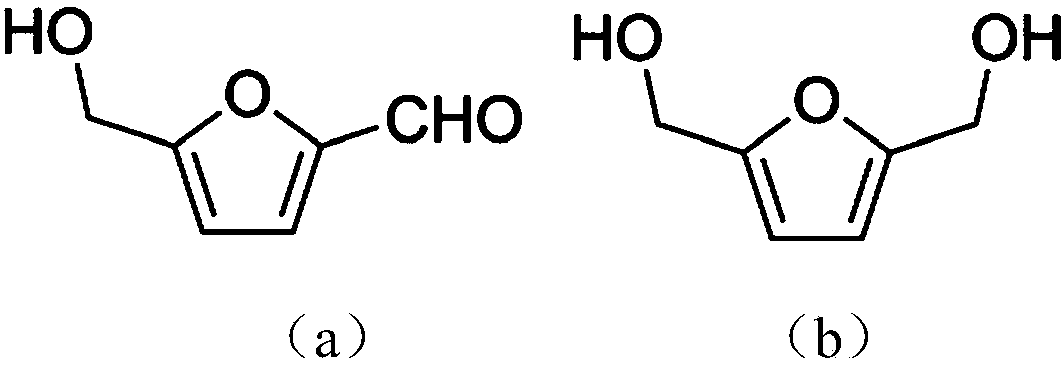
Selective synthesis method for 2, 5-dihydroxymethylfuran
A dimethylolfuran and selective technology, applied in the direction of microorganism-based methods, methods using microorganisms, biochemical equipment and methods, etc., can solve the difficulty of increasing product separation and purification, reduce economy and practicability, The catalytic system is complex and other problems, to achieve the effect of repeated use, easy separation and purification, and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Pichia guilliermondii (Meyerozyma guilliermondii) SC1103 domestication and activation: Pichia guilliermondii SC1103 strains were inserted into yeast extract powder glucose (YPD) liquid medium (1% yeast extract, 2% peptone) containing 5mM HMF and 2% glucose), after culturing at 30°C and 200r / min for 12h, the above-mentioned bacterial suspension was inserted into fresh YPD liquid medium containing 5mM HMF with an inoculum size of 2%, and then incubated at 30°C and 200r / min Under culture for 12h. Subsequently, the above-mentioned bacterial suspension was successively introduced into YPD liquid medium containing 10 and 15 mM HMF with an inoculation amount of 2%, and similar steps were adopted to gradually acclimatize. Inoculate the above-mentioned acclimatized Pichia montagii SC1103 strain into the YPD liquid medium containing 15mM HMF, cultivate it at 30°C and 200r / min for 12h, and inoculate the above-mentioned bacterial suspension with an inoculum of 2%. Put into fresh Y...
Embodiment 2
[0029] Immobilization of Pichia mongolica SC1103: Weigh 2.5g of sodium alginate into a Erlenmeyer flask, add 100mL of distilled water, and heat to dissolve. After the solution was cooled to room temperature, the cells acclimated and activated in Example 1 were added at a concentration of 200 mg / mL-500 mg / mL (based on the wet weight of the cells). Add 0.2M CaCl drop by drop with a sterile syringe 2 After standing in the solution for 4 hours to solidify, the solution was washed with Tris-HCl buffer (100 mM, pH 7.2) for 3 times, and stored in a refrigerator at 4°C for use.
Embodiment 3
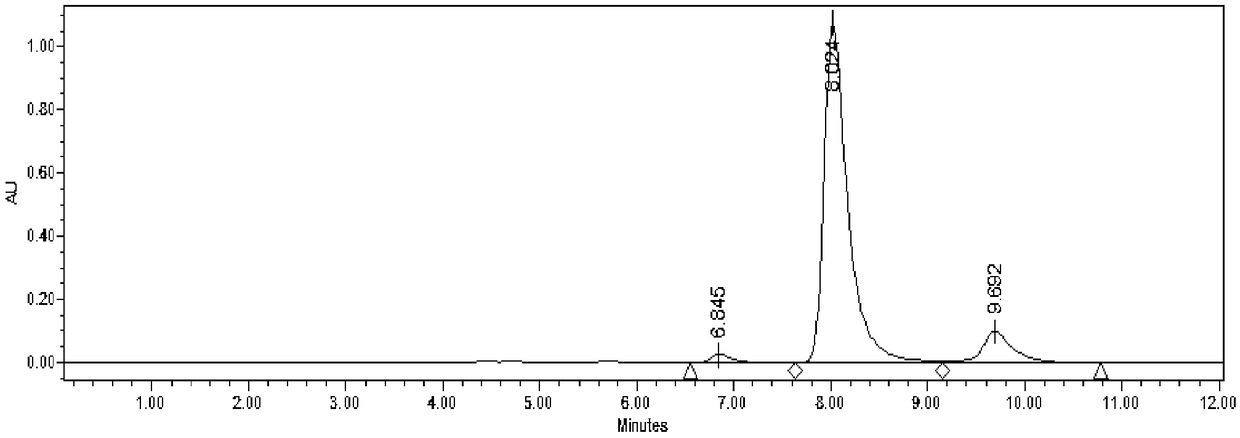
[0031] Add 50mM HMF and 30mM glucose to 4mL acetate buffer (50mM, pH 5.0), mix well, add the immobilized Jiyemeng prepared in Example 2 at a concentration of 20mg (wet cell weight) / mL (buffer solution) Pichia pastoris SC1103 cells were reacted at 35°C and 200r / min. The reaction was monitored by liquid chromatography ( image 3 ). After 9 h, liquid chromatography analysis showed that the yield of DHMF was 63%, and the selectivity was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com