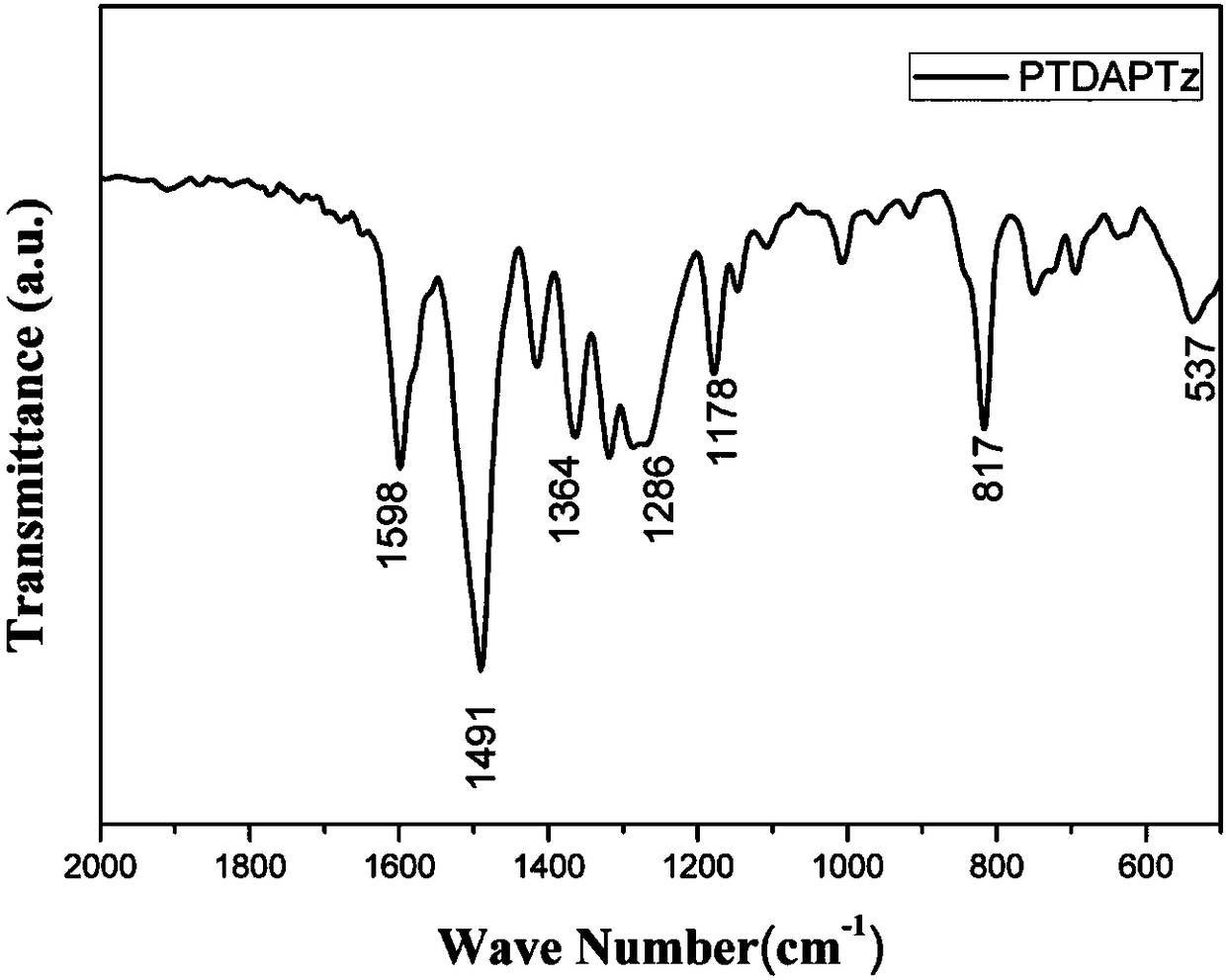
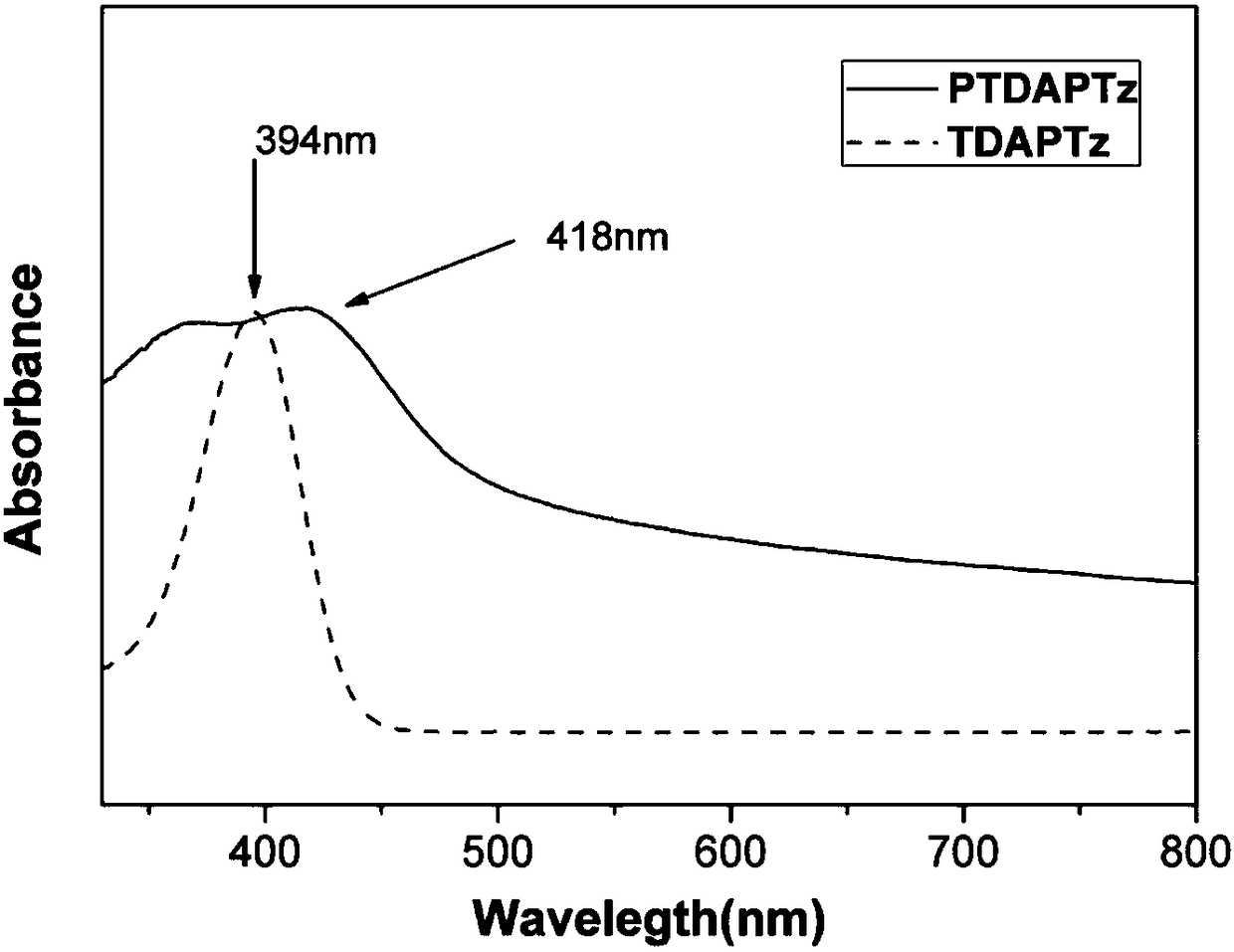
Novel conjugated microporous organic polymer and synthesis and application thereof
A compound and reaction technology, applied in the field of poly[1,3,5-tritriazine] conjugated microporous organic polymer materials and their synthesis, can solve problems such as lack of reports, and achieve no attenuation and superior discharge specific capacity. Cyclic stability, effect of improving electron transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Poly[1,3,5-tris(4-dianilinophenyl)triazine]
[0053] Synthesis of 4-dianilinobenzonitrile: Add sodium hydride (1.5g) to a 250mL three-necked flask that has been washed and dried in advance, then add 50mL of anhydrous N, N-dimethylformamide (DMF), and stir thoroughly Dissolution, a large number of bubbles will be generated during the dissolution process. After no bubbles are generated, add diphenylamine (5.1g) and slowly raise the temperature to 110°C, the reaction solution turns green tea green, then slowly add p-fluorobenzonitrile (4.5g), the reaction solution immediately turns dark red, react for 12h reached the end of the reaction. The reaction crude product was washed several times with dichloromethane and water, extracted and dried over anhydrous magnesium sulfate, and filtered. The extract was desolventized by a rotary evaporator, and separated and purified by column chromatography to finally obtain 4.98 g of 4-dianilinobenzonitrile with a yield of 61...
Embodiment 2
[0061] The synthesis of 1,3,5-tris(4-dianilinophenyl)triazine monomer is the same as in Example 1.
[0062] Synthesis of poly[1,3,5-tris(4-dianilinophenyl)triazine] (PDAPTz): Dissolve 0.5g of monomer in 30ml of chloroform, slowly add an oxidizing agent with a molar mass 4.5 times that of the monomer without Ferric Chloride Powder. The reaction was carried out at 60°C for 12h under the protection of nitrogen. After the reaction, a large amount of methanol was added to precipitate the product, and then filtered. The filter cake was alternately washed three times with methanol and water, and the obtained filter cake was vacuum-dried at 60° C. for 24 h. The product was obtained as a yellow solid powder.
Embodiment 3
[0064] The synthesis of 1,3,5-tris(4-dianilinophenyl)triazine monomer is the same as in Example 1.
[0065] Synthesis of poly[1,3,5-tris(4-dianilinophenyl)triazine] (PDAPTz): Dissolve 0.5g of monomer in 30ml of chloroform, slowly add an oxidizing agent with a molar mass 4.5 times that of the monomer without Ferric Chloride Powder. The reaction was carried out at 20°C for 48h under the protection of nitrogen. After the reaction, a large amount of methanol was added to precipitate the product, and then filtered. The filter cake was alternately washed three times with methanol and water, and the obtained filter cake was vacuum-dried at 60° C. for 24 h. The product was obtained as a yellow solid powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com